|
They are the
commonest, represents about 80% of all intracranial vascular
malformations. The exact pathogenesis is not known. A genetic factor has
been postulated; incidence is of one seventh of that of aneurysms. A male
preponderance is reported by almost all studies.
Pathology:
An AVM is a cluster of congenital arteriovenous
communications without intervening capillaries; the arteries and veins
are tortuous and dilated.
In most, the AVM is visible in the cortex. It fans out
subcortically.
They are more commonly supratentorial, particularly in the
parietal lobe; middle cerebral, posterior cerebral, and anterior cerebral
territories are involved in declining frequencies.10% of them are
infratentorial.
They derive blood supply from one or a combination of
vessels.
Those supplied by the epicerebrals (perforators from the
pial vessels) are confined to the cortex and are drained by cortical
veins.
Those supplied by the transcerebrals (major the parenchymal
vessels) are wedge shaped with its apex reaching the ventricles and
drained by superficial and deep veins.
The centrally located AVMs mostly receive feeders from the
anterior as well as the posterior circulation.
They grow apace with the growth of the brain.
In the presence of large draining veins, and the arterial
feeders are submerged within the brain parenchyma and the AVMs present as
SOLs with mass effect.
Some may be so compact and resemble a cavernoma.
Most have a gliotic core with a nidus and a gliotic wall
forming a ‘pseudocapsule’.
Calcification is not uncommon.
"Cerebral proliferative angiopathy”
(CPA) as a clinical entity has been described lately. CPA may be a
diffuse network of densely enhancing vascular spaces with intermingled
normal brain parenchyma and regarded as separate from “classical ” brain
giant AVMs in angioarchitecture, with low risk of bleed. Because normal
brain is interspersed with the abnormal vascular channels increasing the
risk of neurological deficit in aggressive treatments which does not seem
to be indicated. The discrepancy between the large size of the nidus and
the small shunting volume, the absence of flow-related aneurysms, the
presence of diffuse angiogenesis (eg, transdural supply, progressive
arterial occlusion), and the small calibre of a multitude of feeding
arteries and draining veins were the angiographic hallmarks of this
disease.
Natural history:
Growth of the AVMs occurs in about 20% because of repeated
hemorrhages, gradual dilatation of the vessels and recruitment of new
supply.
In the elderly, especially small AVMs with a single feeder
may diminish in size and on occasions, disappear.
In an unruptured AVM, the incidence of first bleed and the
annual rebleed is about 4%.
The annual mortality rate due to an AVM is 1%, with the
mortality at the first bleed being 10%. The morbidity with each bleed
occurs in 20-30% per episode of bleed, with long term morbidity being
2.7% per year. It has been reported that, in a patient presenting with
seizures, there is a 25% chance of the first bleed within 15 years,
whereas in patients presenting with a bleed, the possibility of a second
bleed was 25% in the next four years, and that of a third bleed is
25%within one year of the second episode.
Studies suggest that only 34% of patients with AVM remained
symptom free; 26% become symptomatic and partially disabled; 11% are
severely disabled.
Untreated posterior fossa AVMs carries a poorer prognosis.
The risk of bleeding is greater in children.
Clinical features:
Hemorrhage: It is the commonest presentation with an
incidence of about 70%. Unlike an aneurysm, AVMs bleed, more frequently
during sleep and it is unrelated to stress, trauma, or hypertension.
It is widely believed that they tend to rupture during
pregnancy; but there is no convincing evidence.
Children bleed more often and the risk declines after the
age of 40 years.
Small AVMs, because of the higher pressure in the feeding
artery, are more at risk.
The posterior fossa and the periventricular AVMs are more
likely to bleed.
There is a higher risk of second bleed in the first year
following a bleed.
High arterial pressure, suggest a higher risk. Smaller the
feeding arterial segment, and the smaller AVMs will have high-pressure
feeders.
Single venous or deep venous drainage, or venous obstruction
suggests a high risk as a result of high flow arterial feeders. The risk
is less in cases of peripheral or mixed venous drainage and in the
presence of an angiomatous change, because of resultant dilated cortical
and leptomeningeal vessels with low flow.
Associated aneurysms are due to mechanical or venous outflow
obstruction and suggest a high risk.
Seizures: It is the second commonest (about 30%) and
associated with subclinical bleed in about 7%. The average age of onset
is 25 years. They are more common with large, superficial, high flow
AVMs.
Arterial steal and resultant ischaemia, gliosis around the
lesion, and the mass effect (due to venous ectasia and retrograde dural
sinus hypertension resulting in hydrocephalus or raised ICT) are the
possible causes for the seizure.
Focal neurological deficit: About 10% of AVMs present with
focal deficit alone and about 25% with seizure or hemorrhage in addition.
The deficit may be due to arterial steal, or mass effect or
hydrocephalus.
Headache: The exact nature of mechanism of headache in
unruptured AVM is not known. It is often seen in AVMs with dural or pial
component.
|
Other features:
In large AVMs, the scalp veins may enlarge, and a thrill associated
with a bruit over the neck may be detected. Retinal angiomas may be
present.
Posterior fossa angiomas may cause trigeminal neuralgia.
High output cardiac failure, especially in children, may
be the presenting symptom occasionally.
Investigations:
CT scan –may suggest a nidus as a low
density within the hematoma (nidus sparing sign). A serpiginous
enhancing lesion with an early draining vein; associated hypoperfused
areas may be evident as low-density areas.
MRI scan –T1 and T2 images may show areas
of flow void; associated hemorrhage including subclincal hemorrhage and areas of cortical atrophy and hypo perfusion are better seen.
MRAngiography and 3D CT may outline the AVM; better suited for follow up studies.
Digital angiography is still the imaging mode of choice. A detailed study of the arterial
feeders, the nidus and venous drainage is mandatory.
SPECT and functional PET scanning are
useful for assessment of cerebral perfusion.
Grading of AVMs:
|
|
|
|
|
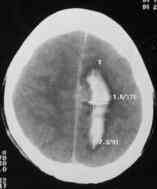
Bleed
due to pericallosal AVM-CT
|
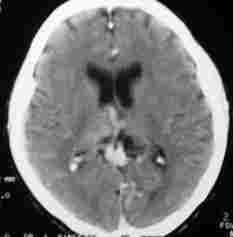
Midline AVM-CT
|
|
|
|
|
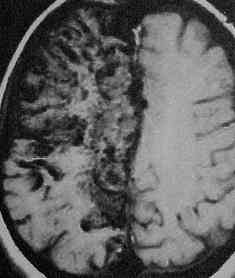
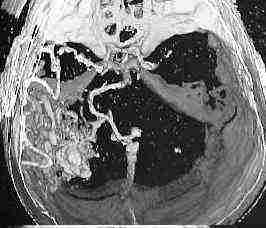
Giant AVM-MRI
|
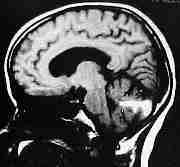
Vermian AVM-MRI
|
|
|
|
|
3D CT--temporal AVM
|
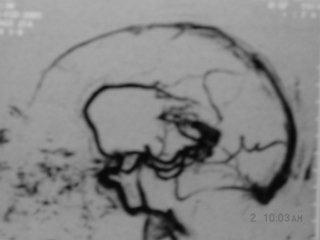
Midline AVM-
MRangiography
|
|
No ideal grading system exists. Spetzler and Martin
grading system (1986) is widely followed, but ignores arterial feeders.
There are 5 grades, arrived at adding the scores. Grade 1 has the best
prognosis and grade 5 has the worst.
Spetzler and Martin grading system:
|
Size of
AVM
|
Eloquence
of adjacent brain
|
Venous
drainage
|
|
Small (<3cm)
1 point
|
Non-eloquent 0
point
|
Superficial only
0 point
|
|
Medium (3-6cm) 2
points
|
Eloquent 1 point
|
Deep 1 point
|
|
Large (>6cm)
3 points
|
|
|
Management:
The aim is to compete obliteration of the AVM on follow up
angiography with no morbidity.
Surgical excision, endovascular procedures and stereotactic
radiosurgery
are the accepted modalities. Conventional
radiotherapy, electrothrombosis, and cryosurgery have not been accepted
in modern practice.
The strategy for a given patient must decided on the
patient’s age and associated conditions, the AVM’s size, site and the
venous drainage etc, and the available facilities and experience.
Surgical excision:
This remains the gold standard; other modalities are
considered only if a safe surgical excision without any long-lasting
morbidity is not feasible. Ideally, cortical AVMs in non eloquent sites
are best treated with surgery.
Surgery is usually delayed for a few weeks (as the rebleed
risk is much less unlike in aneurysms) unless the hematoma requires
emergency evacuation.
Large, high flow AVMs with multiple deep feeders may need to
be embolized before surgery. Some prefer to do it in stages without
embolization. Intraoperative embolization is not popular anymore.
|
A generous
craniotomy is advised.
Associated dural component, if any, should be excised. Any
injury to an adherent vein while opening the dura must be
avoided.Prominent landmarks at surgery are the large arterialized
veins, which need to be protected until the arterial feeders are
coagulated.Bleeding vein may be controlled with gelfoam and cottonoids
and dissection should be continued.
Intermittent hypotension helps on occasions.
As the major feeders are coagulated, the malformation
shrinks. Clipping a feeder shrinks the draining vein whereas clipping
the arterialized vein produces venous engorgement.
Temporary clipping helps in differentiation of the feeders
from the arterialized veins, which, perhaps, is the most important part
of the surgery.
Presence of hematoma helps in delineation of the
malformation and the adjacent gliotic ‘pseudocapsule’ offers a plane
for dissection. Such gliotic areas are encountered, more often, in
deeper areas.
Dissection is kept close to the malformation. As the
superficial feeders are secured, the deeper ones appear to
collateralize and coagulation may be difficult. Use of gel foam and
judicious use of hypotension help.
In case of persistent oozing from the bed, a residual
nidus must be looked for.
Recent advances in anesthesia, laser photocoagulation,
evoked potential monitoring, facilities for intra operative DSA and
cortical mapping have contributed in total excision of these lesions.
Stereotactic localizing helps in deep seated AVMs.
|
|
|
|
|
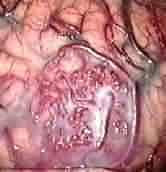
Parietal AVM at surgery
|
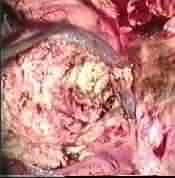
Post excision at surgery
|
|
|
|
|
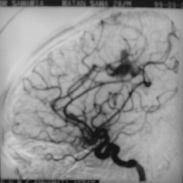
Pericallosal AVM-angio(lat)
|
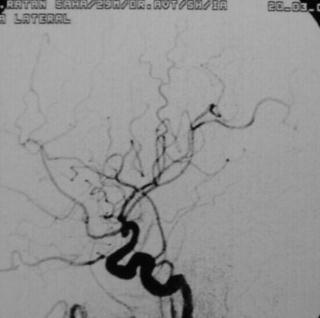
Post Excision -angio (lat)
|
|
|
|
|
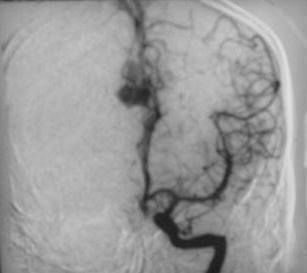
Pericallosal AVM-angio AP
|
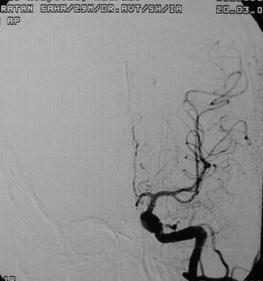
Post excision- angio AP
|
|
In the early postoperative period, brain swelling may be
troublesome. Perfusion pressure breakthrough syndrome is, most often,
blamed. The rapid restoration of perfusion to a chronically ischemic area
with dilated vessels, which is not able to respond to the increased flow,
is assumed to be the cause. They are more likely in patients with CT
evidence of hypoperfusion and / or atrophy. Many studies suggest
hemorrhage from a residual nidus is the cause for the brain swelling.
Stereotactic Radiosurgery:
Conventional radiotherapy has no role.
Radiosurgery using Gamma knife or Linear accelerator is
found to be effective either in a single sitting or repeated sittings.
It is indicated in (1) Small (<2cm) AVMs in eloquent
areas. (2) Poor surgical candidates and in patients who are not willing
for surgical excision. (3) Post surgical inaccessible residual AVMs. Deep
seated AVMs are ideally treated with radiosurgery.
A success rate of 80% at two years of follow-up is claimed.
Limiting factor, in addition to the small size of the AVM,
is the risk of bleeding which persists (3-4%) during the latency period
of 2 to 3 years till the AVM gets obliterated, and may even be enhanced
due to change in hemodynamics. Permanent neurological deficit due to
delayed radiation necrosis occurs in 1%.
Embolization:
Embolization of the nidus or the feeders as definitive
treatment, or as a part of the multimodality approach, prior to
microsurgery or radiosurgery, is getting popular. However, at the present
time, despite the recent technical advances, rate of complete
obliteration of the nidus with embolization alone is low, about 20% in a
recent study. The procedure carries a 20% risk of hemorrhagic and
ischemic complications.
Multimodality
Treatment:
Although
it is difficult to make generalizations about specific uses of
multimodality treatment, such treatment does appear to play a helpful
role in larger lesions. It is done as either a planned maneuver,
typically with embolization followed by surgical resection or
radiosurgery, or as an unplanned maneuver where one treatment modality
fails and a second treatment modality is necessary to obliterate the AVM.
This can occur in situations such as residual AVM after subtotal surgical
resection or resection of an AVM after incomplete radiosurgical
treatment. The aim is total obliteration of the AVM.
|