|
Approximately 0.5-1% of all
primary brain tumors and 15-20% of all intraventricular masses are
colloid cysts.
They may cause sudden death or
longstanding symptoms from obstructive hydrocephalus.They
are still associated with considerable morbidity and mortality.
In 1858,
Wallman first reported colloid cyst. Dandy accomplished the first
successful resection of a colloid cyst in 1921.
Pathology:
Although these tumors are
considered congenital, their presentation in childhood is rare. They
usually present in the middle age.
The origin of these cysts continues to
be uncertain. Remnants of paraphysis, diencephalic ependyma, invagination
of neuroepithelium of the ventricle, or the respiratory epithelium of
endodermal origin are other etiologic possibilities.
|
Colloid cysts are thought to
enlarge through increases in their contents. This process is postulated
to occur in several ways. The epithelial lining of the cell wall
secretes a mucinous fluid. In addition, cyst cavities filled with blood
degradation products and cholesterol crystals have been reported.
Colloid
cysts are slowly growing lesions, probably of endodermal origin, and
usually founding the anterior third ventricle close to the foramen of
Monro, but other locations, such as, the roof of the third ventricle,
the columns of the fornix, or the choroid plexus, are possible.
Their
fibrous walls are lined with simple or pseudostratified epithelial
cells. Their shape is either flattened cuboidal or low columnar, and
they rest on a thin capsule of collagen and fibroblasts. The cysts are
mucin secreting and ciliated.
|
|
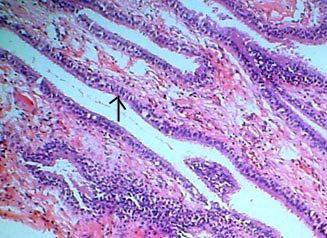
|
|
Colloid
cyst (H&E): cyst wall is lined by cuboidal to
columnar epithelium(arrow), supported by delicate
collagenous stroma
|
|
The Cells are
periodic acid-Schiff (PAS) positive and stain positively for S100 and
negatively for glial fibrillary acidic protein (GFAP), vimentin, and
neurofilament. The stromal wall stains positively for vimentin. Contents
of the cyst are usually greenish and of variable viscosity.
Clinical features:
Symptoms are usually caused by
constant or intermittent hydrocephalus. Headache related to
position of the head and sudden drop attacks are typical. Colloid
cysts have been the cause of sudden death due to obstruction of CSF flow
or hypothalamic disturbance of cardiovascular control.
The
majority of colloid cysts are detected in the work-up of milder
symptoms: Headaches (68%), Gait disturbances (47%), Disturbed
mention (37%), Nausea (37%), Blurred vision (24%), Incontinence (13%),
Tinnitus (13%), Seizures (10%), Acute deterioration (10%), Diplopia
(8%)
Signs
detected include: papilloedema (47%), gait disturbance,
hyperreflexia, positive babinski sign (21-32%), 24% have a normal
examination.
With
the advent of MR and CT imaging an increasing number of colloid cysts
will be incidental findings.
|
Diagnosis:
CT scan demonstrates a usually hyperdense (iso-and
hypodense are also possible), and may enhance with contrast.
MRI enables visualization the same typical
features, But with better anatomical detail and with delineation of
venous anatomy.
Differential diagnosis includes other tumors in
the region such as hamartomas, astrocytomas and ependymomas, and benign
cysts of the choroid plexus. The latter are adjacent to the
plexus, usually in the lateral ventricles, hypodense and non-contrast
enhancing on CT.
|
|
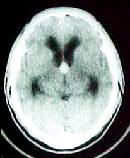
|
|
Colloid cyst-CT
|
|
Treatment:
Common
modes of treatments are stereotactic aspiration, microsurgical
extirpation and endoscopic fenestration of the cysts.
For
small (<0.5), asymptomatic cysts that do not cause hydro-cephalus can
probably be followed without treatment, although data on possible
deterioration are lacking. This is, however. controversial. This
approach does restrict the patient to life long follow up.
Ventricular shunting:
Traditionally
bilateral shunts have been advocated. The rationale was an
assumption that the cyst occluded both foramina of Monro. It is,
however, possible that CSF flow is actually interfered with in the
posterior part of the ventricle. A single shunt would thus
suffice. Shunting carries a risk of sudden deterioration if
dysfunction occurs, and does not alleviate symptoms caused by pressure on
the fornices or the hypothalamus. Shunt revisions for infection or
malfunction are common.
Microsurgical excision:
Transcallosal
approaches are feasible with normal ventricles. The main risks are
venous infarction from interference with bridging veins, and damage to
the pericallosal arteries. Traction on the gyrus cinguli may
produce (usually transient) mutism. Disconnection syndromes are not
detectable following a small (<2.5 cm) callosotomy, which is made
starting 1-2 cm behind the genu. An operation can be carried out
through a 1.5 cm callosotomy. Special tests may, however,
demonstrate minor deficits.
The other approaches apart from the transcallosal that are
available for lesions in this area are the transcortical,
transventricular and the subfrontal. The subfrontal approach is
used for tumors that arise inferiorly and compress the third ventricle
from below. The transcortical approach involves doing a frontal
craniotomy usually on the right side, cortical incision down to the
ventricle and then locating the foramen of Monroe. The
transcortical route cannot be used when the ventricles are normal or
narrow and requires a lot of brain incision and retraction. The
foramen of Monroe on both sides cannot be visualized if necessary.
The transcallosal approach which was first
employed by Dandy and later on by others is a direct midline approach to
either or both lateral ventricles and the third ventricle. There is
no cortical incision and only retraction. The small opening made in
the anterior part of the corpus callosum (1.5 to 2.5 cms) does not cause
any disconnection syndromes. Occasionally a frontal cortical vein may
have to be sacrificed to get adequate retraction of the frontal lobe and
this may lead to convulsions or to venous infarction of the frontal
lobe. The size of the ventricle is inconsequential in this approach
which can be used after the patient has been shunted.
The steps of the operation described is the way the author
does and is most comfortable with.
The patient is positioned supine with the head elevated 20
degrees. Three pin fixation is not used, only a head ring is used.
It should be made sure that the head is not tilted to the
left or right to a great extent. A question mark skin
flap is turned with the medial limb on the midline. Two thirds of
the medial limb is anterior to the coronal suture and one third is behind
it. The posterior end is curved downwards towards the zygoma for 7
to 8 centimetres. The other skin flaps that may be used are
bicoronal and horseshoe.
A free bone flap is turned with the medial end on the midline
to expose only the lateral edge of the superior sagittal sinus (SSS). If
there is a small ridge of bone lateral to the sinus, this must be
ronguered or a Kerrison punch can be used to remove the inner
table. This is necessary to avoid excessive retraction of the
frontal lobe and the need to work under a ledge of bone. Some
surgeons recommend going across the midline and others do not. The
advantage of going across the midline is that a little more space
may be available The author feels that the space available is
determined by the SSS rather than by the extent of bone removal.
The disadvantages are the potential injury to the SSS when the whole of
it is exposed and the chance of pressure on the sinus during retraction.
This pressure can lead to venous stasis and raised intracranial pressure
during surgery or venous infarction of either frontal lobe. The
ledge of bone left over the SSS prevents this pressure.
After applying the hitch stitches on the dura, the dura is
opened as a flap hinged towards the sinus. The lateral extent
of the dural opening should be only about 2 cms from the midline so that
the retracted frontal lobe stays under the dura and does not get hitched
against the cut dural margin. In some, due to cortical veins entering
the SSS, the entire anteroposterior extent of the dura cannot be
opened. The restriction is usually posterior and the anterior two
thirds of the dura can be comfortably opened and this exposure is
adequate. 3 to 4 cms of longitudinal exposure of the frontal lobe is all
that is necessary for retraction.
In many patients one or more cortical veins will be seen in
the area exposed and they will be entering the SSS. As far as
possible, the retraction should be done between these veins. An
extra 3 to 5 mms of the vein can be mobilized by dissecting the arachnoid
around the vein in the cortex. If absolutely essential the smallest
vein may be sacrificed. The vein should be anterior to the coronal
suture. In the authorís experience sacrifice of a vein has been seldom
necessary.
On gentle retraction of the frontal lobe, the falx will be
seen. There may be adhesions between arachnoid granulations and the
falx or sinus and these have to be released. In patients, in
whom the brain is tight and the ventricles are enlarged the right frontal
horn can be tapped and CSF let out. When the ventricles are normal
in size 20% mannitol can be given in a dose of 5 ml per kg body
weight. It is advisable not to give mannitol when the ventricles
are enlarged as at the end of surgery there will be excessive shrinkage
of the brain due to a large quantity of CSF being let out during
surgery.
The frontal lobe is retracted initially using a one cm
retractor. The angle of the microscope has to be changed to 60
degrees to the right during this step till the falx is seen well.
The microscope angle is then changed to have direct vision of the
interhemispheric fissure. The arachnoid in the fissure is dissected
and CSF is let out. Further retraction is carried out only after
letting out the CSF which leads to further relaxation of the brain.
A 2 cm retractor is now used. In most cases the two frontal lobes
are easily separable but in some sharp dissection may be required.
Sharp dissection lessens the chances of injury to the cortex. A
branch of the pericallosal artery may be seen coursing in the cortex on
one or both sides. The cingulate gyri may be densely adherent to
each other and in some the fissure may be angled to the left or
right. It is extremely important to identify the cingulate gyri and
not mistake them for the corpus callosum.
The two pericallosal arteries will now be seen. The
arachnoid between and around the arteries are dissected and the corpus
callosum will come into view. The pericallosal arteries can be
displaced to one side or the dissection can be carried out
between the two arteries. This will depend on the anatomy seen in
each individual patient and there is no hard and fast rule. Rarely
a single pericallosal artery may be seen. Occasionally a small cortical
branch may have to be sacrificed in order to mobilise the pericallosal
arteries and get enough space to expose the corpus callosum.
The corpus callosum will be white in color with a few small
arteries and veins coursing over it. The cingulate gyrus should not be
mistaken for the corpus callosum as then the entry into the ventricle
becomes difficult and confusing. An incision is made in the corpus
callosum for a distance of 1.5 to 2.5 cms, depending on the type and size
of the pathology in the ventricle. The callosum is relatively
avascular and the incision is deepened the ependyma will come into
view. A few small veins may be seen coursing over the
ependyma and these can be coagulated. Initially a small opening is
made in the ependyma in order to let out the CSF slowly. The
CSF should not be rapidly let out especially in patients with dilated
ventricles as this will collapse the brain and may lead to the formation
of a subdural haematoma. The ependyma is then opened to the extent necessary.
The next step is to determine whether the left or the right
lateral ventricle has been opened into. This is determined by
locating the choroid plexus and following it forwards. The choroid plexus
as it is traced forwards curves medially and enters the foramen of Munro.
The thalamostriate and septal veins will also be visible. In third
ventricular tumors it really does not matter which ventricle has been
entered into.
The approach to the third ventricle depends on the size and
location of the lesion and whether the lesion has enlarged the foramen
of Munroe. When the foramen of Monroe is enlarged which is so
in the majority of the cases that the author operated upon, the lesion
can be removed by the transforaminal approach. This is the ideal
approach as there is no destruction of neural tissue or sacrifice of
veins. If in case, there is necessity to enlarge the foramen of
Monroe and this is not a common occurrence, one anterior column of the
fomix can be sacrificed and the foramen enlarged anteriorly. This
should be done preferable on the non dominant side. The other way
is to enlarge the foramen posteriorly by sacrificing the
thalamostriate vein. In many instances, it is possible to
enlarge the foramen posteriorly without sacrificing the vein. The
foramen of Monroe may appear narrow on first appearance and there may be
a bulge posteriorly. In these it is best to make a small incision
in the area of bulge, decompress the lesion and then the foramen of
Monroe will open out and the further dissection and removal of the tumor
can be carried out through the foramen of Munro.
|
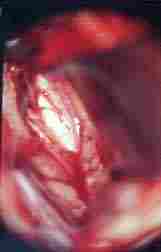
|
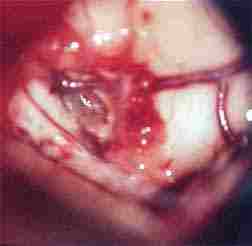
|
|
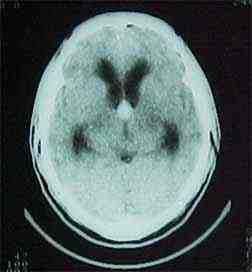
corpus callosum and
pericallosal art exposed
|
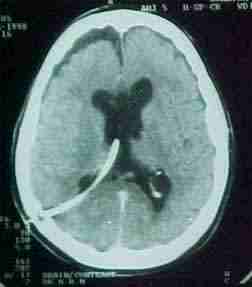
colloid cyst
exposed
|
|
|
|
|
pre op CT
|
post op CT
|
The subchoroidal approach can be used in mid third
ventricular tumors where the foramen of Monroe is not enlarge and there
is no obvious bulge. In this approach, the choroid plexus is
mobilized from the choroidal fissure. This will require
cauterization of the choroid plexus and mobilizing the branches of the
medial posterior choroidal arteries. A microdissector passed under
the tela chloridea will expose the plane of cleavage between the
medial wall of the thalamus and the roof of the third ventricle. The
internal cerebral vein in continuity with the septal vein will
dissect away from the ipsilateral dorsolateral thalamus and will remain
suspended in strands of arachnoid of the velum interposium. The
thalamostriate vein should be sacrificed only when absolutely essential.
The interfomiceal approach is the other option available and
should be used only rarely and in specific instances. The approach should
be strictly in the midline with a midline callosotomy and dissecting
between the two fornices. The fornices are very delicate structures
and can be damaged easily leading to post operative problems.
The ideal lesion in which this can be used is where there is a direct
upward extension of the tumor and the fornices are spread apart by the
tumor. The maximum neural complications occur in the interforniceal
approach and most neurosurgeons do not use this approach except in rare
instances. The complications that can occur are memory disturbances
and a state of mutism.
Complications: The complications that
may specifically occur with the transcallosal approach are (1)
immediate post operative convulsions especially if a cortical vein
has been sacrificed. This is not often seen. (2)
Disconnection syndromes are extremely rare in anterior callosal sections
limited to 2.5 cms. (3) Transient lower limb weakness may occur if there
has been some pressure on the pericallosal artery during
retraction. The other complications like acute hydrocephalus,
transient mutism, memory disturbances and hypothalamic disturbances are
related to the surgical procedure in the lateral or third ventricle and
are not related to the transcallosal approach.
|
Stereotactic
aspiration:
This
new technique was originally claimed to have lower morbidity than
excision, but severe complications have been reported. Initial
success is achieved in approximately 50% of cysts. A 100% recurrence
rate has, however, been reported following aspiration procedures.
This was not surpirising since vital epithelium capable of producing
mucoid was left intact.
Ventriculoscopic
surgery:
|
|
|
|
|
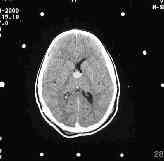
before aspiration
|
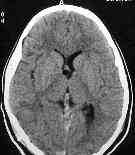
after aspiration
|
|
Endoscopy
is a minimally invasive means of operating in the ventricular
system. Improved instrumentation has allowed the use of flexible
and non-flexible endoscopes for fenestration and aspiration of colloid
cysts, and lately, excision.
Endoscopy
for aspiration remains a treatment with the drawbacks of simple
aspiration. Aspiration with a generous fenestration of the cyst was
described recently. A fenestration should allow continuous emptying of
the colloid produced and thereby avoiding recurrences.
These
methods are recent, and have not yet been available for long-term
follow-up.
Prognosis:
Prognosis
following successful microsurgical removal is excellent. The risks
of microsurgery depend on the skills of the surgeon. Different
results have been reported.
Patients
treated with shunting carry a risk of deterioration when shunts
malfunction and are at risk of shunt infection.
Aspiration
has an unacceptable recurrence rate, and patients treated with aspiration
procedures need to be followed and re-operated when a recurrence appears.
Significant morbidity from recurrence following aspiration has been
reported.
The
natural history of colloid cysts is not well known. An increasing
number of cysts are incidental findings, and will be followed without
surgical intervention. The safety of conservative treatment and
risks of deterioration remain to be established.
Simple
aspiration has been challenged lately while endoscopy is becoming more of
a routine tool in many departments. Its use for colloid cyst
surgery appears to become established, but long term follow-up is
necessary to evaluate its safety in fenestration procedures.
|