|
Management of
tumors of the temporal bone and base of skull is one of the most
challenging problems. The intimate association of these tumors with
the carotid artery, jugular vein and the V through XII cranial nerves
have in the past rendered many patients inoperable.
The development of the infratemporal fossa approach, as
pioneered by Fisch, has allowed the excision of lateral skull base and
petrous apex lesions which were previously deemed unresectable.
These approaches are classified as type Fisch A, B and
C.
TYPE A approach: click for intra operative
video clippings
This approach is used for removal of tumors involving the
jugular foramen and vertical, segment of petrous internal carotid artery,
primarily class C and D glomus temporal tumors. This approach is
also indicated for meningiomas, cholesteatoma involving the internal
carotid artery and petrous apex, for intratemporal neuromas of
cranial nerves IX-XII and for lesions reaching the skull base from below
(Carotid artery aneurysms, glomus vagale tumors etc).
Operative technique:
Surgical highlights:
Retroauriculo – cervico – temporal skin incision
Blind sac closure of external auditory canal
Facial nerve exposed in parotid
Great vessels and cranial nerves exposed in the neck
Subtotal petrosectomy
Permanent anterior transposition of facial nerve
Ligation of the sigmoid sinus
Eustachian tube obliterated
Mandible displaced anteriorly
Internal carotid artery exposed
Jugular foramen and infralabyrinthine space exposed for
tumor removal
Middle ear cleft obliterated with fat and temporal is muscle
flap.
The key point of this approach is the anterior transposition
of the facial nerve, which provides optimal control of the
infralabyrinthine and jugular foramen regions, as well as the vertical
portion of the internal carotid artery.
|
A standard, curvilinear post auricular incision is
extended into the upper neck.
The anterior flap is elevated superficial to periosteium
over the mastoid and deep to platysma in the neck. The external canal
is transected at the bony cartilaginous junction and the flap continued
forward over the parotid for 2-3 cms. The lateral external ear
canal skin is undermined from underlying soft tissues, everted, and
over sewn to create a blind-sac closure of the EAC. The facial nerve is
dissected out in the parotid.
The upper neck is next dissected, vessel loops are placed
proximally around the internal and external carotids and silk ties are
placed, but not yet tied, around the internal jugular vein.
The vagus and accessory nerves are identified as they exit
the jugular foramen and the hypoglossal is noted as it crosses the
carotid bifurcation.
The sternomastoid muscle is dissected from the lateral and
medial mastoid tip and mobilized with the post auricular flap.
A well beveled canal wall down mastoidectomy is next
performed.
The remaining EAC skin, tympanic membrane, malleus and
incus are excised, and the sigmoid sinus is completely
skeletonised.
The entire middle ear and mastoid course of the facial
nerve is identified using cochlear form process, horizontal
semicircular canal and digastric ridge as landmarks.
The facial nerve is decompressed to 270 of its
circumference where possible, from the geniculate ganglion to the
stylomastoid foramen.
The mastoid tip and the bony EAC are quickly removed with
large cutting burr and bone roungeurs while constantly keeping facial
nerve in view.
If there is limited intradural extension, the dura is
opened without injury to the endolymphatic sac.
Tumor is carefully removed from the carotid artery
anteriorly, if necessary. Often, a surgical plane between the
carotid artery adventia and tumor can be identified. When such a
plane is not present and tumor is adherent to the adventitia, residual
tumor is left on carotid and later cauterized.
Deep infralabyrinthine tumor extension may
involve the inferior internal auditory canal, thereby
placing the cranial nerves VII and VIII at risk. At times
labyrinthectomy may be necessary to permit exposure and safe tumor
removal from the IAC.
Whenever possible, the medial wall of the jugular bulb is
left intact, thereby protecting the cranial nerves IX through
XI.
The eustachian tube is obliterated with muscle and facial
plugs.
The surgical cavity is obliterated with abdominal
fat.
The procedure described above is used for glomus jugulare
tumors.
TYPE B approach:
|
|
|
|
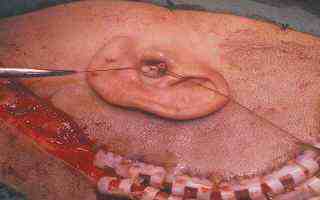
Skin incision and blind closure of EAM
|
|
|
|
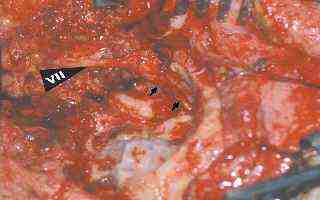
Rerouted 7th nerve
|
|
|
|
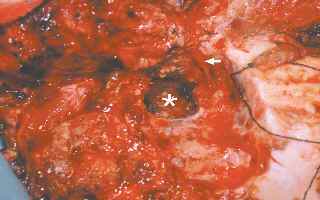
Tumor bed after excision
|
|
|
|
Pre and post OP CT
|
|
In this approach, the skin incision is extended anteriorly,
the zygomatic arch is divided and the petrous carotid artery is
skeletonized. The temperomandibular join is then disarticulated,
the eustachian tube detached anteriorly with associated soft tissue, and
the middle meningeal artery and mandibular nerve divided as needed.
This provides access to the clivus and petrous apex and is
applicable to glomus tumors involving the horizontal petrous carotid
artery, clival chordoma, and congenital cholesteatoma of the petrous
apex.
TYPE C approach:
This is an anterior extension of type B and allows for
exposure of the parasellar region, nasopharynx, pterygomaxillary fossa
and eustachian tube. It has been used primarily for extensive
juvenile nasopharyngeal angiofibroma and radiation failure squamous cell
carcinoma.
The management of intracranial tumor extension depends on
the size and location of the tumor, and the status of the patient.
Small intracranial tumor extension are removed with the jugular bulb
because this is typically the site of dural penetration. The
decision to remove large intracranial extensions is based on the
hemodynamic status of the patient.
Blood loss in excess of 3 liters usually prompts a second
stage approach to total tumor removal.
Post-operative care:
All patients who have undergone infratemporal fossa
dissection are monitored overnight in the intensive care unit for
evidence of hemorrhage or evolving neurological injury.
Postoperative hemorrhage is extremely rare
due to the extensive measures taken to ensure intraoperative vascular
control. However, given the complexities of modern skull base
surgery and the advanced stage in which most skull base tumors present, postoperative
cranial nerve deficits are inevitable. Jacksons reported that
76% of his patients with extensive skull base neoplasms suffered a new
intraoperative cranial nerve deficit, the most common being a glosso
pharyngeal / vagal lesion. Likewise, Spector found that 19% of glomus
jugulare patient suffered a partial or complete VII nerve paralysis
postoperatively. In the later stages of growth, many skull base
neoplasms tend to envelop rather than infiltrate the contiguous cranial
nerves. Consequently, it may be possible to maintain anatomic neural
integrity by microsurgical tumor dissection of the nerves, if the
involved nerves are not intentionally sacrificed during tumor removal.
Because such dissection tends to devascularize the nerve, many patients
will suffer a transient cranial nerve palsy as a result of their surgery
and will require temporary supportive care.
In all cases of facial paralysis, either transient or
permanent, it is essential that adequate corneal protection be provided
by medication, temporary taping, placement of gold weights or
tarsorrhaphy.
Because of the high incidence of transient dysphagia and
aspiration, most patients remain intubated for at least 24 hours or until
they are fully cognizant. In selected cases, tracheotomy and
nasogastric tube feeding may be required for several weeks, particularly
if multiple cranial nerve palsies including X, XI, XII have
occurred. Early vocal cord medialization, either by endoscopic
teflon injection or external thyropalsty, may be necessary to permit
decannulation in those cases with new vagal lesion and severe
aspiration. In rare instances, combined vagal and hypoglossal
injury may lead to permanent tracheotomy and gastrostomy. Except in those
cases with extensive intracrianl extension, cerebrospinal fluid leak
and meningitis are rare due to the multiple layers of protection
offered by EAC and eustachian tube closure along with mastoid cavity
obliteration. When CSF leak does occur as heralded by external
wound leakage or rhinorrhea, initial treatment is bed rest with head
elevated, lumbar drainage, and pressure dressings. If conservative
measures fail wound exploration with possible repacking of the cavity
and/or ventriculo peritoneal shunting may be necessary.
Summary:
The infratemporal fossa approach, in conjunction with the
application of microsurgical technique and improved perioperative care,
has permitted significant advances in lateral skull base surgery.
The glomus jugular tumor is the prototypical neoplasm resected by this
approach, although this technique can be applied to a host of additional
benign and malignant lesions of the skull base. This approach
entails identification and control of the cranial nerves and great
vessels in the neck, anterior transposition of the facial nerve, and
infralabyrinthine petrosectomy. Intracranial tumor extension and petrous
carotid artery involvement remain limiting factors. Significant
morbidity, particularly neurological deficit and hemorrhage, may occur
due to the nature and location of lateral skull base tumors. Recent
advances in preoperative embolization and temporary carotid artery
balloon occlusion have advanced the limits of resection via the
infratemporal fossa approach.
|