|
All anatomic structures of
the orbit can give rise to neoplasia. Fortunately orbital tumors are
very rare. There are over 1500 different tumors that can affect the
orbit. The majority of these tumors are benign. Occasionally, a
malignant tumor may involve the orbit primarily or through spread
from an adjacent or distant tumor. These lesions not only cause
problems because of their proximity to vital structures, but also the
risk of spread to adjacent and distant organs. Direct extension from
contiguous anatomical structures, lymphoproliferative disorders, and
hematogenous metastasis result in orbital invasion.
Surgical
anatomy:
The
orbit in the broadest sense describes the cavity containing
structures essential for ocular function and the bony architecture
that encases them and resembles a pear, with its widest aperture
anterior and narrowing posteriorly. It is an anatomically complex
structure containing the globe, extraocular muscles, fat, vascular,
nerve, glandular, and connective tissues.
The
apex is formed by the optic canal and superior orbital fissure. The
roof is made up of the frontal bone. The maxilla and zygoma form the
floor. The lateral wall is made up of the zygoma and the greater wing
of the sphenoid. The maxilla, the lacrimal bone, and the ethmoid bone
contribute to the medial wall.
The
optic canal is about 10mm long, 5mm wide, and 5mm in height. It
extends anteroinferolaterally at an angle of about 40 degrees to the
sagittal plane from the optic foramen. The upper root of the lesser
wing of the sphenoid forms the roof of the canal. Medial border is by
the sphenoid sinus and the ethmoid air cells. The optic strut, a bone
ridge joining the lesser wing to the body of the sphenoid bone, forms
the inferior lateral border of the canal. The superior orbital
fissure is retort shaped with the broad end placed medially. A
fibrous ring, the annulus of Zinn, surrounds the optic canal and the
medial dilated part of the superior orbital fissure.
The
intracranial archnoid continues as a discrete structure through the
optic canal and fuses with the pia at the globe. At the orbital
portion of the optic canal, the pia and the archnoid are fused
dorsomedially and ventrally with the dura and the fibrous annulus of
Zinn. The intracranial dura continues through the canal as a
dural-periosteal layer and then separates into the dura of the optic
nerve, and the periorbita. At the apex, the six extraocular muscles
oigin from the annulus of Zinn. The levator muscle arises from the
upper medial margin of the annulus, and the superior rectus, lying
immediately beneath the levator, arises from the superior portion of
the annulus. Medial rectus is more medial and inferior. The annulus
loops widely around the nerve, laterally and inferiorly, giving rise
to the lateral rectus which has two heads. The muscles broaden as
they pass forward to form a cone.
The
annulus of Zinn envelops the optic foramen and the medial aspect of
the orbital fissure. The portion of the orbital apex enclosed by the
annulus is called the oculomotor foramen. This foramen transmits the
superior oculomotor, the inferior oculomotor, the abducens, and the
nasociliary nerves. They remain inside the muscle cone. The
trochlear, frontal, and lacrimal branches of the 5th nerve
and the superior ophthalmic vein pass through the orbital fissure.
The
ophthalmic artery, branches off from the ICA, just above the
cavernous sinus. It passes in the optic canal lateral and inferior to
the optic nerve. It provides the major supply to the optic nerve. As
it enters into the orbit, it becomes more medial, and 8-15 mm behind
the globe it gives off the central retinal artery that penetrates
into the medial midportion of the optic nerve to supply the retina.
The primary venous drainage is through the superior and inferior
ophthalmic veins. The intraorbital optic nerve is about 30mm long,
5mm longer than distance from the posterior margin of the globe to
the orbital apex.
On
unroofing the orbit, the frontalis nerve is visible through the
periorbita. On opening the periorbita, the frontalis nerve is seen
overlying the levator and superior rectus muscles. In the same plane,
lies the trochlear nerve, which crosses from lateral to medial above
the optic nerve. The nerve is approached medially between the dorasal
superior rectus and the medial rectus muscles. This obviates
potential trauma to the nerves passing through the oculomotor
foramen. In the orbital apex, the optic nerve is approached laterally
so as not to jeopardize its blood supply.
Clinical
features:
Progressive
proptosis
is the most common symptom. Apical tumors and those within the muscle
cone push the eyeball forwards (axial proptosis). Extraconal tumors
displace the eyeball in the opposite direction. A hyperostosing sphenoidal
meningioma produces prominence of the lateral wall. Pulsatile
exophthalmus in neurofibromatosis suggests a defect in the sphenoid
bone. The tumor may be palpated.
Diplopia
with
limitation of extraocular movements is also common. Commonly, it is be
due to mechanical factors. Muscle infiltration or motor nerve palsies
may also result in diplopia. The eye can be rotated freely by the
examiner in nerve palsies after anesthetizing the conjunctiva.
Progressive
visual loss
may be the presenting symptom in some. It may be transient and only
in certain directions of gaze. The unilateral visual loss may be
detected by the patient at a late stage. Testing for color vision may
detect an early visual defect.
Pain is not
common and is a late manifestation in malignancy. Painful paresis of
one or more ocular nerves point to a cavernous sinus lesion.
Chemosis suggest an
inflammatory lesion or carotico-cavernous fistula (CCF), and rarely
in a malignant lesion. A bruit may suggest a CCF. Intraorbital AV
fistula and hemangioma can also produce a bruit.
Pupillay
abnormalities due to isolated sympathetic or parasympathetic
nerves as they pass through superior orbital fissure are unusual.
Ophthalmoscopic
examination
will demonstrate either papilledema or optic atrophy. Chronic
compression of the central retinal vein redirects retinal blood to
the choroids via a pre-existing system resulting in optociliary
shunts. A retroocular striae may suggest a lesion deforming the
orbit.
Investigations:
MRI is the
imaging of choice. It is particularly valuable in assessing the
orbital pathway because of the high degree of sensitivity of fat
tissue, changes of hydration within the soft tissue, and lack of
ionizing radiation. Gadolinium MRI adds to better delineation.
A CT
may help t o visualize the bony involvement better. Plain x-rays
have become obsolete.
Carotid
angiography helps in evaluation of CCF and orbital AV fistula.
Duplex
ultrasound is useful to assess the hemodynamic flow in the
ophthalmic and retinal arteries.
Management:
The
management of orbital tumors greatly depends on such factors as tumor
type, tumor location, patient age, and vision. However, the
following generalizations can be made.
Orbital
inflammatory syndrome (orbital pseudotumor) may be amenable to
steroid treatment.
If
the orbital lesion is discrete, then surgical excision may be
curative. Examples of discrete lesions include cavernous
hemangioma, unruptured dermoid cysts, neurofibromas and schwannomas.
If
the orbital lesion infiltrates the tissues, complete excision may not
be possible without harming the eye. Examples of infiltrative
lesions include lymphoma, lymphangiomas, and orbital metastases.
Depending
on the type of tumor, further surgery, radiation, chemotherapy or a
combination of the aforementioned treatments may be required.
Vascular
orbital tumors composed of large blood vessels are difficult to
address surgically because of their tendency to bleed. Some
vascular lesions of the orbit are amenable to embolization with the
aid of an interventional radiologist
Surgery:
Restoration
of the proptosed eyeball to is normal position with preservation of
vision and ocular motor function and cosmesis are the main goals of
orbital surgery.
Surgical
spaces in the orbit are used to define the location of the lesion.
The
three surgical spaces are the subpersiosteal, peripheral surgical and
central surgical spaces.
a)
The subperiosteal space potentially exists between the orbital wall
and the periorbita. Frontal and ethmoidal sinus mucocoels, epithelial
tumors arising from sinuses, orbital abscesses, dermoid cysts and
metastases begin in the subperiosteal space before encroaching onto
the deep orbital structures. b) The peripheral surgical space exists
between the periorbita and the extraocular muscles. Lesions involving
the peripheral surgical space are lymphangioma, hemangioma, dermoid
cyst, lacrimal gland tumors, metastatic lesions or orbital varices.
C) The central surgical space is seen posterior to the eye-ball,
within the muscle cone. In the central surgical space cavernous
hemangioma, hemangio-pericytoma, neurofibroma and pseudotumor are the
lesions commonly seen.
The
position within the surgical spaces and the character of the lesion
determine the specific choice. The surgeon must use clinical and
radiographic information to decide on the simplest and safest
approach to the orbital lesion. The approach is designed according to
the location and nature of the lesion. Those with intracranial
extension or involvement of the apex are primarily the responsibility
of the neurosurgeon. Those with paranasal extension require skullbase
approach ideally. Lesions not involving the apex and wholly within
the orbit may be managed by an ophthalmologist or a neurosurgeon.
Three
routes are used in orbitotomy: anterior, lateral and superior
(transcranial). The orbit may be approached by any route or by a
combination of these.
Anterior
approach: The
majority of orbital procedures can be carried out through an anterior
incision in skin or conjunctiva. More commonly, ophthalmic surgeons
use this approach. This approach is useful for biopsy of lesions
anywhere in the orbit or to remove well-defined anteriorly located
tumors. Access can be through conjunctiva or skin. When approached
through skin, the dissection may either extraperiosteal or more
directly through the orbital septum. The main incision sites are
superior, inferior, in quadrants, medial and lateral or directly over
a palpable lesion.
There
are three anterior approaches: transconjunctival, extraperiosteal and
transeptal.
Transconjunctival
approach:
Some
anterior periocular and intraconal lesions can be approached by
direct conunctival incision and dissection. A rectus muscle may be
disinserted to enter the intraconal space and the retractors placed
between the muscle and the globe. In addition, the optic nerve may
accessed by this route where it can be operated upon following
disinsertion of the medial rectus muscle, with lateral rotation and
anterior distraction of the globe. This is a particularly useful
approach to optic nerve sheath decompression for chronic papilledema.
Exraperiosteal
approach:
The
anterior extraperiosteal approach is most useful for lesions
occurring in the peripheral surgical space adjacent to periosteum or
arising from and involving bone. In particular, lesions such as
dermoid cysts are readily accessible by this approach. The skin
incision is usually made just at the orbital rim and carried down to
the periosteum, which can then be incised and elevated. The
extraperiosteal space can then be safety and extensively explored. An
alternative route of access inferiorly can be by means of subsciliary
incision through skin and orbicularis muscle with dissection along
the plane of the orbital septum and incision of the periosteum at the
orbital margin. The entire floor of the orbit can be easily explored.
For
the most part, anterior orbitotomies do not require bony resection.
But some large superior orbital lesions can be more readily accessed
by temporary removal of the superior orbital margin. A clearer view
of the entire superior orbital space can be gained this way. It is
not necessary to transect the supraorbital nerve when operating on
large superior lesions. The nerve can be distracted after unroofing
the bony canal or incising the overlying ligament at the time of
superior orbital exploration.
Larger
explorations through the extraperiosteal space usually require
postoperative drainage with a Penrose drain. It should be cautioned
that the extraperiosteal approach should not be utilized in biopsy of
suspected malignant intraorbital lesions because the periosteum
provides a barrier to regress of malignancies.
Trans-septal
approach:
Trans-septal
route involves entry into the orbit through the orbital septum
leaving the periosteum intact. This approach is indicated for biopsy
of most unresectable orbital malignancies. Anteriorly placed small
tumors can be removed through this route. Incision can be made
anywhere along the inferior orbit, but lacrimal sac must be avoided
medially. Superior incisions have to avoid the supraorbital and
supratrochlear nerves. Skin incision is made over the preseptal
orbicularis within the orbital rim. In the lower lid a subciliary
incision may be used in younger patients. Orbicularis is opened and
separated. Traction sutures are put to promote exposure and
hemostasis. After identifying the orbital septum, gentle pressure is
applied over the upper lid which produces a forward displacement of
the orbital fat and septum. The septum is opened and extended both
medially and laterally. Orbital fat is displaced with a malleable
retractor to locate the lesion. The trans-septal approach can be
accessed through the relaxation lines around the eye.
Lateral
approach:
In 1889, Krönlein first described the lateral orbitotomy approach.
This approach is used less often these days. Lateral orbitotomy
provides the best access to reach the posterior lesions both within
and outside the muscle cone. Ideally, the lesions lateral to the
optic nerve and the apex are dealt with by this approach. The amount
of bony excision can be customized to include more or the
superolateral orbital rim, and even the zygomatic arch when
necessary, depending on the size and location of various lesions.
Most retrobulbar and parabulbar lesions can be handled by an anterior
or lateral orbitotomy alone or in combination.
The
patient is positioned with the head slightly elevated and minimally
rotated in the direction opposite the operating side. Two types of
incisions are advocated to reach the lateral orbit. The Wright incision
(a curvilinear incision, extending from the lateral half of the
eyebrow to the zygomatic arch, anterior to the hairline) allows a
greater access to the lacrimal gland fossa tumors and lesions in the superior
and posterior quadrants. The length of the incision can be adjusted
depending on location and extent of the orbital mass. Dissection is
carried down to expose the periosteum and temporalis muscle. The
Berke incision involves a 3 to 5 cms horizontal incision after a
complete lateral canthotomy. The upper and lower limbs of the lateral
canthal tendon are dissected completely from the lateral orbital rim.
The
periosteum is incised from the superior aspect of the zygomatic arch
to the zygomatioco-frontal process. The periosteum and temporalis
muscle are reflected posteriorly. Stripping the temporalis muscle
from the bony fossa needs blunt dissection. Then the periorbita is
elevated from the inner orbital wall. Separation of periorbita from
the orbital rim to the apex must be done meticulously. After
protecting the globe with a malleable retractor, bony cuts are made
using a saw or chisel. Superior bone cut is above the
fronto-zygomatic suture and inferior cut is along the upper margin of
the zygomatic arch. The bony opening may be enlarged posteriorly to
the depth of temporalis fossa with a rongeur or drill.
The
periorbita is incised antero-posteriorly and a vertical incision is
made to form a ‘T’. Then the lateral rectus is identified and kept
aside by gentle traction suture or umbilical tape. On occasions, one
may have to do deeper intraconal dissections to expose a tumor mass
or to operate on the optic nerve.
For
evaluation of the central surgical space, especially if the lesion is
small one, gentle traction can be exerted on the suture placed into
the stump of the scleral insertion of the lateral rectus muscle. This
maneuver pulls the optic nerve into view without direct pressure by
the surgeon. The short ciliary arteries are readily seen and should
not be torn. It is important to remember that the central retinal
artery enters the optic nerve inferiorly about 10 to 15mm behind the
globe. If the dural sheath is to be opened, it is well to incise it
on its anterolateral surface, well away its vasculature.
Superior
approach: The
superior approach is necessarily the domain of neurosurgeon. Dandy in
1921 laid the foundation for Neurosurgeon’s role in orbital tumors
with transcranial approach. The superior approach indicated in
compound trauma of the orbit and intracranial cavity, decompression
of the optic canal, or for removal of apical or combined apical
intracranial lesions. There are three types of procedures used for
this approach: Panoramic orbitotomy (Fronto-orbital temporal
approach), Frontal approach, Supraorbital approach.
Fronto-orbital
temporal (Panoramic) approach:
This
procedure allows for an en bloc excision of the roof and lateral wall
of the orbit and a wide view of both the orbit and adjacent
intracranial structure. This approach consists of a coronal incision
and removal of the frontal flap in the usual manner followed by
dissection of the temporalis fossa and elevation of periorbita from
the adjacent bone. An incision is then made along the superomedial
wall of the orbit after distracting the frontal lobe from above. The
bony incision is extended along the roof of the orbit to the lateral
margin near the apex, whence the lateral wall and frontozygomatic
process are incised. With removal of the bone a wide view of the roof
and lateral orbital structures is obtained. This approach is
particularly useful for excision of tumors at the apex of the orbit
or for combined intracranial orbital lesions such as tumor of the
optic nerve or sphenoid wing.
Frontal
approach:
With
the patient in the supine position a bicoronal skin incision is made.
A four burrhole frontal bone flap is elevated after a subperiosteal
dissection down to orbital rim. If the tumour is confined to the
orbit an extradural approach is used. The dura is stripped from the floor
of the frontal fossa to expose the orbital roof. Orbital unroofing is
performed initially with a high-speed drill or a chisel and then
rongeurs are used. The optic canal, however, is unroofed not with
rongeurs, but only with a high speed diamond drill. The orbitotomy
extends medially to within 1.5cm of the midline and laterally to
within 1cm of the orbital margin.
When
orbital unroofing is complete, the transparent periorbita displays
the frontal branch of the fifth nerve overlying the superior rectus
and levator muscles. The bony canal must be unroofed and annulus of
Zinn incised apically in the orbit. The best site for incision of the
annulus is medially between the superior oblique origin and the
levator palpebrae-superior rectus origins. The fourth nerve can be
seen coursing over these structures to its site of insertion in
posterior third of the superior oblique muscle. It may be necessary
to transect the fourth nerve in order to deliver and optic nerve tumor.
However, this can be avoided by carefully dissecting the optic nerve
within its dural sheath from the annulus, transecting it
intracranially, and pulling it forward through the annulus into the
orbit, from which it can be removed following transection at the
globe and dissection from adjacent structures. Meningiomas or optic
gliomas are best approached by retracting the superior rectus and
levator muscles laterally. Dissection through the fat is performed
with small retractors and cottonoids. It is possible to dissect the
posterior ciliary nerves and vessels within the orbit and avoid
sectioning them while doing this procedure. After excision of the
tumor the orbital roof is reconstructed with stainless steel mesh or
bone taken from the inner surface of the bone flap.
Supraorbital
approach:
This
approach ensures best exposure of the apical portion of the orbit,
with minimal or no retraction of the frontal lobe. A bicoronal skin
incision is used and the scalp, including the periosteum, reflected
anteriorly. At the superior orbital ridge, the periorbita, which is
continuous with the periosteum, is separated from the surface of the
orbital roof. Two burrholes are placed, one in the midline at the
level of the orbital ridge and the other just behind the arch of
zygomatic process. The two burrholes are then connected superiorly
using a craniotomy to create the bone flap. The bone flap so
fashioned incorporates the superior orbital rim and part of the
orbital roof, thus allowing for an excellent cosmetic closure. A
further 3 to 4cms of the orbital roof may then the removed using
rongeurs. Minimally extradural retraction of the frontal lobe allows
easy access to the superior and posterior orbit.
Combined
approach:
All of the approaches defined above can be used in combinations or
with variations to obtain access to any of the surgical spaces of the
orbit. Widening bony incisions and even removing part or all the
sinus structures to expand the surgical space may rarely be
necessary.
Complications:
Transient
complete or partial lateral and superior rectus palsy occurs in
almost all cases. Improvement usually is seen within several days to
3 to 6 weeks and recovery is complete by three months. Ptosis can be
prevented by gentle retraction of levator palpebrae superioris; if it
occurs, it usually resolves spontaneously. Visual loss can result
from injury to the optic nerve or due to central retinal artery
occlusion while dissecting medial to the optic nerve in the apex.
Perforation of the globe is another potential risk. Postoperative
hemorrhage will cause increasing proptosis, ecchymosis, neuropraxia,
and pain. The rapidity of onset and development varies depending on
the source of bleeding. If the hemorrhage threatens ocular function
(as defined by decreasing vision with an afferent papillary defect)
or if it causes severe pain, prompt relief of orbital pressure is
necessary. CT scan or ultrasonography may help to locate the blood
pool. The decompression can be done through the original incision and
it may be enhanced if necessary by means of alternate routed as for
any decompression.
Orbital
tumors in children:
The
most common childhood tumors are benign and arise from cystic orbital
structures (dermoids) or abnormal blood vessels within the orbit
(hemangioma).
Capillary
Hemangiomas:
Capillary hemangiomas of the orbit are benign vascular tumors,
and are found almost exclusively in children. They are the most
common orbital tumors found in children. Lined by vascular
endothelium and pericytes, these histologic benign lesions manifest
at birth or within the first 3 months of life, enlarge rapidly, and
begin to commence contracting around age 1 year. About 70% of these
tumors spontaneously decrease in size by seven years of age.
There are usually no other associated systemic conditions. CT shows a
large hyperdense, lobulated enhancing mass. Total excision is
impossible. The necessity of treatment depends on whether there are
ocular complications, such as the development of amblyopia (lazy eye)
or strabismus (crossed eye). Most orbital capillary hemangiomas
that cause secondary ocular complications can be treated with
steroids that are either administered systemically, or injected into
the tumor. The prognosis is generally good.
Dermoid
and Epidermoid Cysts: Dermoid and epidermoid
cysts are benign cystic structures which may be present in the orbit,
upper eyelid, or brow. They constitute 5% of the orbital tumors.
They often progress very slowly. CT shows a well circumscribed
low density lesion. Most are treated with surgical excision and the
prognosis is good. Total excision may be difficult.
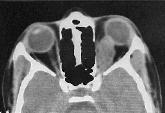
Optic Nerve Glioma: (discussed
elsewhere)
Rhabdomyosarcoma: Rhabdomyosarcoma,
a mesenchymal tumor, is the most common malignant orbital tumor of
childhood. These devastating lesions usually occur in children
younger than age 2 years or older than age 6 years, and they have a
predilection for the superior nasal orbit. The average age of onset
is about 6 years. This type of tumor generally grow very
rapidly, causing proptosis (forward displacement) of the eye.
It may destroy bone and enter adjacent sinuses. CT reveals a
solid homogeneously enhancing tumor. A biopsy is required to confirm
the diagnosis, but the tumor cannot usually be removed surgically
because of its infiltrative nature. Once the diagnosis is
made, the child is treated promptly with radiation and chemotherapy.
Other
malignant lesions include Burkitt lymphoma and granulocytic
sarcoma.
Metastatic
tumors:
Neuroblastomas, Ewing sarcoma, Wilms tumor, and leukemias are the
more common metastatic orbital lesions afflicting children.
Metastatic neuroblastoma is an orbital tumor that may occur in
children with an adrenal gland tumor (neuroblastoma). In fact, 95% of
patients who present with this orbital tumor have a known history of
adrenal gland tumor. These children may present with proptosis
of one or both eyes and conjunctival or eyelid hemorrhage. These
tumors are usually treated with combined radiation and chemotherapy.
Orbital
tumors in adults:
|
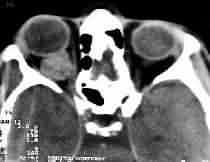
Cavernous
hemangiomas:
They are the most common benign orbital tumor. They are well
capsulated. Histologically, large blood-filled, endothelial-lined
spaces with fibrous interstitial tissue and smooth muscle are discerned.
CT reveals a well outlined hyperdense lesion with minimal
enhancement. Phleboliths (calcifications) may be seen. It is
isointense in ion with minimal enhancement. Phleboliths
(calcifications) may be seen. It is
isointense in T1 MRI and significantly hyperintense to fat
on T2. These lesions usually are well tolerated by the patient and
managed by conservative therapy and reassurance, unless visual
acuity or field loss is found. Total excision is possible.
Meningioma: They
arise from optic nerve dural sheath or periorbita. Sheath
meningiomas (5-6% of all orbital meningiomas) arise from
meningothelial cells present in the meninges covering the optic
nerve. Middle aged females are more frequently involved. More
commonly, they arise within the intraorbital optic nerve sheath
than within the canal. Optic nerve sheath meningioma is the most
common orbital apical tumor and is the most common orbital tumor
with intracranial extension. (However, it is more common for an
intracranial meningioma to extend into orbit. Fibrous dysplasia,
giant aneurysms, and encephalocoeles can also extend into the
orbit). More often, they originate at the cranial end of the optic
canal between the optic nerve and carotid artery and secondarily
extend into the orbit. The tumor may grow extradurally, resulting
in early proptosis or subdurally, resulting in early visual loss
and papilledema; proptosis occurs at a later stage. In combined
intra and extradural types there is progressive visual loss with
proptosis. Some of them are bilateral. Lesions near the optic
foramen or the optic canal produce sclerosis of the bone or
widening of the optic canal and they may be seen in a plain x-ray.
Sclerosis of the optic foramen in a skull x-ray rules out a glioma.
CT and MRI delineate the tumor from the optic nerve.
The
treatment is controversial as the tumor is slow growing and the
natural history is variable. Radical excision will invariably cause
loss of vision and is better delayed until significant visual
compromise. Excision of the tumor involving the apex will also
result in a frozen eye. Subtotal excision and radiotherapy may be
an acceptable alternative.
|
|
|
|
|
|
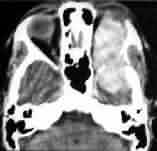
Periorbital meningioma-CT
|
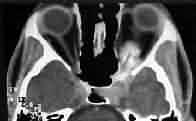
Optic sheath meningioma-CT
|
|
|
|
|
Optic nerve glioma-CT
|
Cavernous Hemangioma -CT
|
|

|
|
|
Schwannoma with intra-cavernous extension-MRI
|
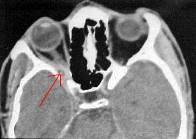
Cysticercosis-CT
|
|
|
|
|
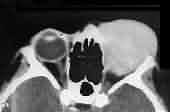
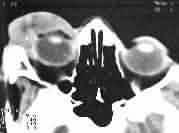
Rhabdomyosarcoma-CT
|
Lymphangioma-CT
|
|
In
the younger patients, these tumors are aggressive and radical
excision may be justified.
Periorbital
meningiomas
arise from the archnoid cells in the superior orbital fissure and
involve the upper lateral quadrant of the orbit. Ectopic archnoidal
cells within the perineurium of a peripheral nerve have been
postulated as another source. Those involving the superior orbital
fissure can not be removed without damaging the nerves passing
through the fissure; subtotal excision and radiotherapy will be more
acceptable.
Lymphoma:
It
accounts for about 10% of all orbital tumors, usually as a
manifestation of systemic disease. It can occur anywhere in the orbit
and may mimic a pseudo tumor. Circumscribed forms usually involve the
lacrimal gland and the diffuse form affects the muscle cone. There
may be bone destruction. CT shows variable morphology. On MRI it is
hypo intense with gadolinium enhancement.
Pseudotumor:
It
is a localized inflammatory disease that mimics a tumor. There is no
associated local or systemic cause. The patient usually presents with
rapidly progressing, painful proptosis. They may be self limiting
with or without visual impairment. Recurrences are common. It is
usually unilateral. Bilateral ones must be differentiated from
Grave’s disease, or systemic collagen disease. CT reveals multifocal
involvement around tenon’s capsule, thickening of extraocular
muscles, and enlarged lacrimal gland. MRI shows nonspecific
widespread involvement. Occasionally, the diagnosis is by a biopsy.
40-60 mg/day of prednisolone over weeks to months is the usual
treatment.
Hemangiopericytoma: It is from
the pericyte of a capillary and usually well encapsulated.
Occasionally it is infiltrative. The patient presents with slow
painless proptosis.
Lacrimal
gland tumor: 5% of the orbital tumors are the epithelial
tumors of the lacrimal gland. Majority of them are inflammatory.
50% of neoplastic ones are mixed ones (painless proptosis) and the
other 50% are carcinomas, mainly, adenoid cystic carcinoma (painful
proptosis). CT shows a mass in the superotemporal quadrant. A period
of conservative therapy with anti-inflammatory drugs is indicated
when inflammatory swelling is suspected. Progressing lesion or the
presence of bony involvement warrants an en bloc excision. Radical
excision may be considered in malignancy.
Benign
nerve sheath tumor: It could be neurofibroma,
schwannoma, or malignant sneurilemmoma. And may or may not be
associated with neurofibromatosis. They occur commonly in the
superolateral compartment and present with proptosis, more marked in
schwannomas than in neurofibromas. Total excision is possible;
however, total excision is impossible with plexiform neurofibroma.
Mucocoeles:
They
are a common cause of proptosis. They result from expansion of the
sinuses secondary to blockage. Pyococele results when infected. A
smooth bulge in the bony wall of the ethmoid or frontal sinus is
characteristic. Complete removal including the sinus mucosa and
restoration of drainage is essential.
Lymphangioma:
It
is slow growing tumor that infiltrates the orbital tissue.
Spontaneous intratumoral hemorrhage can produce episodic proptosis.
Total excision is impossible.
Fibrous
histiocytoma: This is the most common mesenchymal orbital
tumor. It is a benign, slow growing, unencapsulated infiltrative
tumor of the orbit, and may become malignant. CT reveals a high
density mass. Total excision is advised.
Dermoid
cyst,
optic nerve glioma, granuloma, and sarcomas are other
benign orbital tumors in adults.
Metastatases: It is the
most common malignant tumor of the orbit. In adults, carcinomas of
breast and the lung are the most common source. Though proptosis is
common, schirrous carcinoma of the breast may produce retraction of
the globe. CT morphology is variable.
Adenocarcinoma,
squamous cell carcinoma, lymphosarcoma, and mixed malignant tumors
are other common malignant orbital tumors.
|