|
In 1817, Parkinson, in the classic ‘Essay on the Shaking
Palsy’, described a novel condition that now bears his name. He described
the cardinal manifestations: tremor, bradykinesia, gait and postural
disturbance. Charcot, who was so impressed by Parkinson’s description
that he called the constellation of symptoms Parkinson’s disease,
expanded description of the disease in the nineteenth century - named paralysis
agitans by Marshall Hall in 1841.
Parkinson’s
disease accounts for 75% of cases of Parkinsonism .
The current surgical population is distinct from those
treated before the use of L-dopa. Today’s surgical candidate is more
likely to have true idiopathic PD and likely has been treated with
several medications before surgery. For many, surgery is performed to
eliminate l-dopa-induced dyskinesia, an iatrogenic condition that did not
exist before the introduction of L-dopa therapy, and one that may be
unavoidable no matter how L-dopa is administered.
Physiologic basis of
surgery:
The development of primate models of PD and the refinement
of single cell microelectrode recording have resulted in a vastly
improved understanding of PD pathophysiology. The following three points
are essential to understand current surgical approaches:
1. Although an intact
pallidothalamocortical loop is necessary to exhibit tremor, lab data
suggest that tremor activity is probably generated in the ventrolateral
nucleus of the thalamus. These data support the clinical observation that
lesions or high-frequency stimulation in this region best treat
parkinsonian and essential tremor.
2. The striatum modulates
activity in the globus pallidus pars interna(GPi) both directly and
indirectly, via the globus pallidus pars externa(GPe) and the subthalamic
nucleus(STN). In PD, loss of dopaminergic input to the striatum leads to
a reduction in direct pathway activity and an increase in activity along
the indirect pathway, changes that result in STN and GPi hyperactivity.
The output from GPi to
ventrolateral thalamus is inhibitory, reducing the excitatory thalamic
input to supplementary motor areas that are critical to the normal
execution of movement. Thus hyperinhibitory outflow from GPi may account for
the negative symptoms of PD(i.e., rigidity and bradykinesia). Reduction
of this excessive inhibitory outflow, by lesioning or chronically
stimulating GPi or STN, can reverse these symptoms.
3. With time, differential
sensitivities of the direct and indirect pathways to L-dopa therapy may
occur. This imbalanced response to exogenous L-dopa may result in
choreiform movements that characterize L-dopa induced dyskinesias. It is
not completely clear, however, why ablating GPi eliminates this condition.
The advent of axial computed tomography and then magnetic
resonance imaging has altered stereotactic technique. Employing these
imaging modalities with modern stereotactic frames, the position of deep
brain targets can now be sited directly, not estimated on
ventriculograms. Nevertheless, current MR imaging does not always
demonstrate deep brain structures (such as specific thalamic subnuclei)
with sufficient resolution for it to be the sole means of targeting.
Moreover, MR imaging is prone to distortions. Although typically small,
they can be large enough to affect targeting for these types of
procedures. Therefore some type of intraoperative physiologic
confirmation is essential for performing successful movement disorder
surgery.
Microelectrode, semimicroelectrode and macroelectrode
techniques have been described. Microelectrode recording yields the
highest quality localizing information, so that lesioning or DBS
implantation can be performed with greatest confidence. Concerns that the
increased number of trajectories employed with microelectrode recording
increases the risk of hemorrhage have not been substantiated; however it
is questionable whether the extra time, effort and expense of performing
these are necessary to achieve results.
Neuroablative procedures:
The goal of neuroablation is to disrupt irreversibly an
abnormally functioning structure (usually a nucleus), leaving volitional
movement intact. Ideally the lesion is just large enough to achieve the
desired result but not so large as to cause collateral damage. During the
first half of the twentieth century, neurosurgeons lesioned numerous
sites throughout the motor system, searching for the ideal target.
Lesions within the ventrolateral thalamus and GPi have yielded the best
results. Many lesioning techniques have been employed – radiofrequency
thermocoagulation is the most commonly used modality because of its
reliability and simplicity.
Ventrolateral
thalamotomy: Hassler and Reichert are credited with making the initial
foray into the ventrolateral thalamus, reporting improvement in both
tremor and rigidity. Thalamotomy thereafter became the favored surgery in
the pre-dopa era because the surgical response is instantaneous and more
easily monitored than the response to pallidotomy. A number of surgeons
have reported excellent long-term and short-term tremor suppression after
thalamotomy, although contemporary authors do not report significant
improvements in rigidity or bradykinesia. Tremor may be suppressed with
lesions anywhere within the ventrolateral thalamus, but the ventralis
intermedius subnucleus of the ventrolateral thalamus is considered by
most to be the ideal target. The surgical mortality rate for thalamotomy
is less than 0.5%; typically resulting from intracerebral hemorrhage.
Morbidity may vary from 9-23% - but has decreased due to improved
lesioning technique, and patient selection. Dysarthria and contralateral
hemiparesis are the most common adverse events. Speech difficulties are
even more prevalent in those undergoing bilateral procedures. Dystonia,
hemiballism and athetosis have also been reported. In most instances, PD
causes a resting tremor that diminishes with action, making this symptom
one of the least disabling, and its elimination does not improve the
functioning of the patient. Therefore it has given way to pallidotomy
which addresses disabling PD symptoms such as rigidity, dystonia and
dyskinesia.
Pallidotomy: in
1960 Svennilson et al reported superior results with pallidotomy by
placing the lesion posterior, inferior and lateral to the previously
described pallidotomy site. This was exemplified in the work done by
Laitinen et al in 1992. Posteroventral pallidotomy dramatically improved
rigidity, bradykinesia, tremor and ambulation in the majority of moderately
advanced PD patients. Most important was the discovery that pallidotomy
reduced or eliminated dyskinesias induced by dopa. It has been proved
that pallidotomy reduces or eliminates dopa-induced dyskinesias,
rigidity, muscular spasms and off state dystonia in patients with
idiopathic PD who are still dopa responsive, but suffer from motor
fluctuations. Tremor may also improve, but not as consistently as with
thalamotomy. It improves the off state function and prolonged on states
that are free of dyskinesia. Up to 60% improvements with effects lasting
for 2 years have been reported.
The most frequent complication is visual field deficit (14%)
– due to extension of the lesion into the optic tract. Injury to the
internal capsule, facial paresis and cognitive deficits may occur.
Complications increase with bilateral procedures – speech, swallowing and
cognition.
Gamma
pallidotomy and thalamotomy: Leksell originally
conceived the gamma knife to perform stereotactic neuroablative
procedures without open surgery. But its use was limited by inadequate
imaging. With high resolution MRI gamma knife surgery for neuroablation
has become a reality. Only a few cases of thalamotomy and pallidotomy
have been reported; the rate of improvement is lower than that for conventional
procedures (50%). Disadvantage being that the patient has to wait for
weeks to months to notice any positive changes. Therefore clinical
efficacy/potential complications cannot be determined at time of surgery.
|
Deep brain
stimulation:
The idea for long-term DBS arose from the observation that
high-frequency stimulation of the ventrolateral thalamus arrests
tremor, a phenomenon that is employed for physiologic localization of
the thalamotomy target. If one can arrest tremor with stimulation, why
lesion? The advantages of DBS are – a reversible, functional lesion is
made in lieu of a permanent anatomic one, stimulation parameters can be
adjusted over time to maintain tremor suppression in the event of
disease progression. The disadvantage being cost and maintenance of the
device. The deep brain electrode is placed through a burr hole. As with
the lesioning procedures the patient is awake and his neurologic status
can be assessed throughout. Once placed correctly, the electrode is
anchored to the skull. As the electrode is flexible, it can move with
the brain and maintain its anatomic location. Each electrode has four
contacts. Stimulation can be performed in monopolar bipolar fashion.
Once acceptable stimulation parameters have been identified for the
patient, they are programmed into the implantable pulse generator,
which, similar to a cardiac pacemaker is placed within a subcutaneous
pocket below the clavicle and connected to the electrode via wires that
are tunneled beneath the skin. Stimulation parameters may be adjusted
at any time employing a transcutaneous programmer. A magnet may be used
to turn the stimulator off at night or to switch between two stimulator
settings.
The mechanism through which DBS achieves its functional
results is presently unclear. Stimulation may create a depolarization
block, jamming the signals emanating from an abnormally functioning
structure, although this is unsupported by more recent findings.
Alternatively antedromic and orthodromic propagation of the depolarization
may affect distant structures that transmit to or receive impulses from
the stimulation target.
Thalamic deep brain stimulation:
Initially Benabid et al employed
thalamic stimulators contra lateral to previously successful
thalamotomies in patients with bilateral tremor to reduce the risk
associated with bilateral thalamotomy. The results demonstrated that
thalamic DBS achieves tremor suppression with an equivalent efficacy to
thalamotomy.
The tremor suppression was dramatic
and significant. Tasker retrospectively compared DBS and thalamotomy
performed in one venter, and found that initial tremor suppression was
comparable in both, but tremor recurrence was higher (15% versus 5%) in
the thalamotomy group. Tremor recurrence necessitated reoperation in
the thalamotomy group, while it could be resuppressed with adjustments
to the stimulation parameters in the DBS group.
Neurologic complications were less
frequent in DBS group and could be controlled with stimulator
adjustments. Painful dysesthesias secondary to stimulation of the
ventrocaudal nucleus (lies immediately posterior to the ventralis
intermedius); dysarthria; and dystonia are the predominant
complications. Hemorrhage rates and congnitive difficulties may be less
frequent.
|
|
|
|
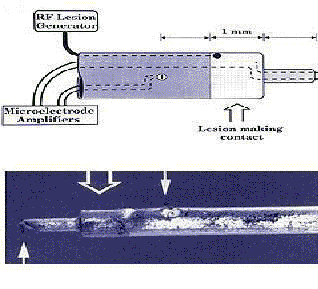
Radio frequency lesion generator and electrode.
|
|
|
|
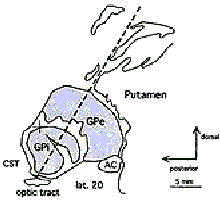
Ideal
target location for Posteroventral Pallidotomy
|
|
|
|
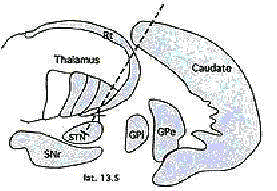
Ideal target location for Subthalamic
nucleus.
|
|
|
|
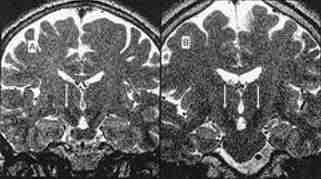
subthalamic nucleus as seen on T2W
|
|
Pallidal stimulation:
In 1994 Siegfried and Lippitz reported their initial
experience performing bilateral pallidal DBS implants. Significant motor improvements
comparable to pallidotomy were achieved. Rigidity and dyskinesias were
the most responsive symptoms. Gait difficulties are helped less. Ventral
stimulation eliminates dyskinesias and rigidity but worsens akinesia
whereas dorsal GPi stimulation reduces akinesia but may worsen
dyskinesia. This made pallidal stimulation to have a narrow therapeutic
window.
Subthalamic nucleus stimulation:
Limousin et al and Pollak et al demonstrated that bilateral
STN stimulation is safe and improves akinesia and gait abnormalities.
These two are the most disabling and medically resistant sequelae of PD,
they do not improve after lesioning or stimulation in the thalamus
or GPi. STN ablation is not done because lesions there cause a
medically resistant, violent hemiballism. Additional benefit of STN DBS
is the reduction in requirement of l-dopa after surgery. Krack et al
reported improvements in tremor and off state dystonia in large series on
patients. Dyskinesia is reduced secondarily by medication reductions.
Preoperative evaluation:
Patients should not be considered surgical candidates until
all reasonable medication strategies have been tested. Initially a
movement disorders neurologist, who confirms the diagnosis of PD and
makes sure that the patient is truly medically refractory, evaluates the
patient. The surgeon then evaluated by a neurosurgeon who decides which
surgery would best addresses the patient’s symptoms. Patients must have a
recent MRI to rule out concurrent neurologic disease – multiple
infarctions, cerebral atrophy. Detailed neurosphsychological testing is
performed to rule out co-morbid dementia, a common sequela of PD that can
be worsened by surgery. If an elderly patient stands to gain significant
functional benefit from alleviation of surgically treatable symptoms and
meets all the selection criteria, surgery is offered. But is the patient
has advanced PD, is bedridden, l-dopa unresponsive and contracted, no
surgery is offered.
Selecting the proper procedure:
1. Those who still respond to
L-dopa therapy but less consistently are asymmetric in their symptoms,
and plagued by motor fluctuations and dopa-induced dyskinesia are
excellent candidates for pallidotomy/stimulation. Patients with off state
dystonia or muscle cramping will also benefit.
2. In patients with bilateral
dyskinesia, staged bilateral pallidotomy/bilateral stimulators may be
considered.
3. In the rare PD patient who
is truly tremor predominant thalamotomy or thalamic stimulation is the
choice.
For those with significant gait disturbances, a predominance
of axial symptoms and medically resistant akinesia, bilateral STN or GPi
stimulators is the only viable options.
|
Procedure
|
Tremor
|
Dyskinesia
|
Rigidity
|
Bradykinesia
|
Off state dystonia
|
Freezing
|
Gait disturbance
|
|
Thalamotoy /thalamic stimulation
|
+++
|
+
|
+
|
-
|
+
|
-
|
-
|
|
Pallidotomy
|
++
|
+++
|
+++
|
++
|
+++
|
+
|
+
|
|
Pallidal DBS
|
++
|
+++
|
+++
|
++
|
+++
|
++
|
++
|
|
STN DBS
|
++
|
++
|
+++
|
++
|
++
|
+++
|
++
|
Refinements in the
understanding of the anatomic, physiologic and the neurochemical
underpinnings of Parkinson's disease have greatly contributed to this
renewed interest in neuroablative surgery and are giving rise to newer,
more sophisticated therapies. Modern stereotactic technique permits
targeting with millimeter accuracy. Deep brain stimulation has been
introduced as an alternative to neuroablation. It is hoped that cellular
transplantation or virally mediated gene transfer therapies that seek to
restore or preserve function will soon be clinically
viable.
|