|
An accumulation blood between the dura and the archnoid
constitutes subdural haematoma. The classical view is that the subdural
space is a preexisting space although, electron microscopy suggests no
evidence of naturally occurring space at the dura-arachnoid junction.
Subdural hematoma (SDH) may accumulate on any site of the
inner surface of the skull. Longitudinal cerebral fissure is a rare
location.
Acute subdural hematoma (ASDH):
Etiopathogenesis:
The incidence varies from 5 to 22% of severe head injuries;
more common in men and those above 40 years of age compared to
extramural haematoma.
The ASDH results from high speed rotational acceleration
injuries of the brain in relation to the fixed dural structure and can
occur during deceleration also. This causes either tearing of surface or
bridging veins between the cortical surface and venous sinuses. The
bridging veins are 1-2 cm long and occur in the largest in the frontal
and parietal regions.
If it is associated with extensive area of lobar contusion
and ICH, this combination is often referred to as a burst lobe.
If it is above 100ml and the only cause of increased ICP, it
is called true ASDH; it occurs mainly in small children, elderly
and alcoholics. Almost ten times more common is a group of small volume
hematomas, with disproportionate mass effect due to intrinsic brain
injury.
The commonest site is the anterior temporal region but
inferior frontal, parietal, bilateral haematomas are encountered. There
may be a combination of EDH SDH & ICH also.
Rare causes are anticoagulants and coagulopathies. Rarer
still, are those due to rupture of an aneurysm.
Ischemic brain damage occurs below the
haematoma. Haematoma as well the underlying brain releases vasoactive and
neurotoxic substances. It is also found that autoregulation of brain is
disturbed following the injury causing impaired cerebral blood flow.
Decoupling of cerebral edema of the underlying brain aggravating the
effect of direct actions of the neurotoxic and vasoactive substances. The
substances are thought to be ghetamate, aspartate, free radicals,
platelete aggravating factors, etc. These secondary auto – destructive
procedure play to greater role in the clinical manifestation, and
prognosis of the patient than the damage due to primary injury. This was
substantiated by the low morbidity & mortality (20%) in cases where
the arachnoid matter was not breached. Ischemic brain damage is not
reversible even after removing the clot.
Diffuse axonal injury may coexist since the
etiopathogenesis of ASDH and DAI being the same. The clinical picture and
the outcome is dominated mainly by DAI.
Clinical features:
They result from raised ICP. Loss of consciousness from the
time of injury suggest underlying brain injury rather than ASDH alone;
the patient deteriorates rapidly. In 60% brainstem damage is found with
bilateral babinski sign, respiratory disorders, pulse and systemic blood
pressure fluctuations.
|
Diagnosis:
Differentiating between EDH and ASDH is usually
impossible. Early onset of impaired consciousness suggest ASDH. CT scan
helps. and is the choice of imaging.
CT scan done immediately after the trauma may not reveal
the hematoma, but reveals the signs of increased ICP, such as
narrowing of the ventricles, and obliteration of sulci.
|
|
|
|
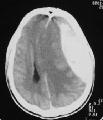
CT- large ASDH
|
|
Plain Xray - A limited value in presence of CT
scan as it gives little information and delaysdiagnostic evaluation
except in case of associated depressed fracture and localizing CSF
fistula.
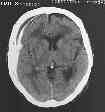
CT scan - The most important
diagnostic aid. ASDH – appear as a crescentric (cancavoconvex) mass of
increased attenuation adjucent to inner table prominent surround edema,
brain contusion.
|
|
|
|
CT- shallow ASDH
|
|
MRI scan is
more sensitive and helpful in isodense haematoma & to defect
parenchymal injury and DAI. Disadvantage takes too long to perform
& has too many restriction.
Management:
|
|
|
The mode of
treatment is influenced by
|
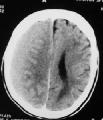
CT- ASDH
|
A. Contribution of ASDH to the overall neurological status
of the patient & the part responsible by associated brain injury.
B. Associated raised intracranial pressure. In condition
where all the potential spaces have been exhausted, decompressive
procedure may play a significant role to reduce ICP.
C. General condition and neurological status of the patient.
After aggressive resuscitation if the patient remains flacoid and brain
stem reflexed are absent, it will not make any contribution by performing
surgery. Patient with no/or minimal neurological deficit do better after
surgery.
D. Size of haematoma :- More than 5 mm thick ASDH
contributing significantly to the mass effect & shift have good
surgical outcome. Smaller SDH with minimal mass effect in a neurologically
intact patient may not require surgical swelling.
E. Age : >65 year old patient do not do well ever with
timely and adequate surgery. Their mortality rate equating with those
managing non surgically.
Thus decision to operate involves a critical analysis of the
contribution of the hematoma to the overall condition of the patient.
In case of large hematomas, surgical evacuation and
securrring the bleeding cortical vessels is mandatory. If required,
bone flap may be emoved. Post operatively, aggressive monitoring and
treatment of brain injury
is warranted.
Prognosis:
It is usually poor in ASDH and depends on the primary brain
injury. Despite medical efforts mortality is about 80%. Most of the
survivers have protracted convalescence and effects of post traumatic
syndrome and about 25% will develop seizure disorders.
Chronic subdural hematomas (CSDH):
Etiopathogenesis:
The incidence varies from 1-2 per 100,000 people per year.
Over 75% occur in patients over 50years of age. 25% to 50% of the
patients have no significant history of head injury. Chronic alcoholics,
epileptics, and those with coagulopathy are more prone. Reduction in ICP
like after shunt surgery in infants and children also predispose to SDH.
Initial hemorrhage from a torn bridging vein, following head
injury (often trivial), may be small and asymptomatic.
The clot lyse (after about 60 hours); there is angioblastic
invasion of clot (4-14 days). The clot breaks down and vascular sinusoids
appear in the capsule(2-3 weeks), which become well developed (3-4 weeks)
prior to liquefaction of the clot (4-6 weeks).
Some undergo spontaneous absorption, some clots get
organized, and some enlarge.
The organized clot gets surrounded by a covering membrane.
On the dural side efforts at absorption of the clot lead to the formation
of vascular and often pigmented fibrous tissue, whereas the deeper layer
adjacent to archnoid is thin. Compaction and fibrosis of the membranes of
both sides occur (1-3 months). The membranes become fused consisting of
mature fibrous tissue (3-12 months) and proceed to calcification and
ossification (about 1 year).
The exact mechanism of enlargement of the hematoma is
not known.
1) Osmotic gradient between the hematoma and the CSF space
facilitates the enlargemet.Various theories exist:
Gardner (1932): The capsule acts as an osmotic
membrane with CSF diffusing into hyperosmotic
hematoma.
Zollinger and Gross (1934):
The flow across the membrane occurs as a result of an increase in osmotic
pressure from a breakdown of hemoglobin molecules in red
cells.
Gitlin (1955): The albumin/gamma globulin and
albumin/total protein ratios in the hematoma are higher than in serum.
Because albumin is not found within red cells, the albumin has to diffuse
across the membrane.
Weir (1971): There is no significant
difference in osmolality with increasing age of the hematoma and no
significant difference in osmolality of blood and hematoma.
Sato and Suzuki (1975): The capillary
endothelial cells of chronic SDH capsule have cytoplasmic protrusions and
fenestrations which are associated with high permeability and permit
passage of protein moieties into the hematoma.
Itoh (1978): The fibrionlytic enzymes in the
hematoma membrane enhance the chance of recurrent hemorrhage into the
hematoma cavity.
Yamashima (1984): The most important
factor for the development of Ch. SDH exists in the vessls of the capsule
which have a marked proliferation potential and a fragile nature; the
endothelial gap junctions of macrocapillaries in the outer membrane of
SDH play a role in the leak of blood, causing enlargement of SDH.
2) Recurrent bleed from the outer membrane is a feasible theory.
The content near the outer membrane is often shaggy and brighter in
color. This also explains waxing and waning of the symptoms in patients
with ch.SDH.
3) Periodic bleeding from a venous stump has been suggested
by Furtado.
4) Low ICP has been suggested as a cause by Kopp.
5) Leakage of CSF from a rupture neighboring arachnoid
granulations into the hematoma has also been blamed.
There are three types:
Type I -CSDH with a visible inner membrane: It
is an expanding lesion.
Type II -ASDH in chronic healing stage: It is
not an expanding lesion; lacks a visible inner membrane.
Type III -CSDH of hemorrhagic type: CSDH in which minimum
amount of fresh blood is added to a maximum amount of xanthochromic
fluids.
Clinical features:
The symptoms are variable. Impaired consciouness (53%),
hemiparesis (45%), papilledema (24%), dysphasia (14%), 3rd nerve palsy
(11%), and hemianopia (7%) are the common signs. Rare symptoms are
parkinsonism, and monoparesis.
|
Diagnosis:
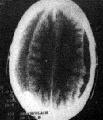
CT scan is the imaging of choice.
After 3 weeks the majority of SDH will be hypodense and assume a
lenticular appearance; because of recurrent hemorrhage, some may have
heterodensity.
|
|
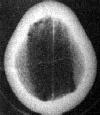
|
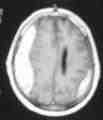
|
|
MRI scan delineates the lesion better.
|
CT- Bil.
CSDH in varying stages
|
CT- Bil
CSDH
|
MRI- Bil.
CSDH
|
Management:
Nonoperative:
In selected patients, with minimal signs and shallow SDH in
a scan, nonoperative management with bed rest, cortico-steroids, and
diuretics has been successful. But the treatment is prolonged, more
expensive than surgical evacuation, and often unsuccessful.
Burr holes:
This is the most common mode of evacuation. Some prefer to
place a drain to prevent a recollection. Some advocate intrathecal
infusion of saline for reexpanding the brain in cases where the cortex do
not surface after evacuation. Many feel such measures are of no use.
Craniotomy:
It provides access to solid components and the membrane
thereby reduces the risk of recollection.
Twist-drill holes:
This may be performed at the bedside in an emergency. A
catheter is passed into the subdural space and connected to a closed
drainage system.
Complications:
Rapid evacuation can cause brain shift and brainstem
hemorrhages.
In bilateral hematomas, both should be evacuated simultaneously,
otherwise the remaining hematoma can cause a rapid brain shift.
Seizures in the postoperative period are reported in up to
11%.
Recurrence:
It has been reported that 78% of the post operative CTs show
residual collection. However, evacuation of only a portion of the
hematoma produces clinical improvement and the residual collection will
gradually resolve.
True reaccumulation has been reported in 8-45% of cases
according to various reports.Recurrence was reported to occur more often
(37%) following craniotomies than in burr-hole patients (20%).
Post operative re-evacuation is possible by needle
aspiration or reoperation, if the patient deteriorates. Some may require
a craniotomy and excision of the subdural membrane. Some suggest complete
obliteration of the subdural space into epidural space; the subdural
pocket is exteriorised so that it is in continuity with subgaleal space
through a limited craniectomy. Some claim no difference with or without
any of these procedures.
Recurrent SDH in a patient with shunt system may warrant
blocking the system temporarily.
Outcome:
75% of patients resume normal activities. the preoperative
neurological status is closely related to the outcome. Size of the
hematoma does not influence the outcome.
|