|
Choroid plexus papilloma
(CPP) was described by Guerard in
1832, and Perthes described the
first successful surgical removal in 1919.
They are rare
intraventricular tumors that arise from choroid epithelium and
account for less than 1%% of all intracranial
tumors.
They are more
common in children than in adults, with a mean patient age of 5.2
years.
20% of them occur
in patients younger than 1 year, and 85% occur in those younger than
10 years.
In utero detection
has been described. They may be discovered
at birth. They account for approximately 40% of pediatric tumors that
are present within the patient's first 60 days of life.
Overall, a 4:1 preponderance
of choroid plexus papillomas to carcinomas
is seen.
No distribution
by race has been described.
They
have been associated with von Hippel-Lindau syndrome, the Li-Fraumeni cancer syndrome (an autosomal dominant
syndrome characterized by a mutation in the TP53 gene), and
the Aicardi syndrome.
One theory of the
etiology involves the presence of
simian vacuolating virus No. 40
(SV40)–related viral DNA.
Pathology:
They may arise wherever a choroid plexus exists.
Tumoral
distribution varies between pediatric and adult patients. In
children, most CPPs (80%) are located in the lateral ventricles.
About 16% of papillomas are found in the
fourth ventricle, and 4% are found in the third ventricle. In those
aged 0-10 years, the relative incidence of third ventricular papillomas approaches 30%.
The fourth ventricle is the most common location of CPPs
in adults.
CPPs occasionally can be bilateral or multiple.
Interventricular extension can occur with a CPP, unlike
other interventricular tumors. Although uncommon, interventricular
extension through the foramen of Munro, cerebral aqueduct, or foramen
of Luschka of Magendie
is a helpful diagnostic sign.
CPPs can arise in the cerebellopontine angle, secondary
to direct extension from tufts of choroid protruding through the
foramen of Luschka. Extension into the
foramen magnum may occur, with possible brainstem compression.
Grossly, these tumors
are dark red in color with a globular outer surface and an even,
gritty cut surface, the latter owing to calcification. They are often are associated with a vascular
stalk connected to the choroid plexus, allowing mobility within the
ventricular system.
|
Histologically, the tumor
resembles normal choroid plexus. It consists of delicate
papillary formations, each made up of a layer of columnar or
cuboidal epithelium resting upon a tenuous vascular connective
tissue stroma. The nuclei are usually circular or ovoid and
the tumor monomorphic and benign. Bone
formation and neuromelanin production may
occur, but these are extremely rare. Rarely, the tumor
secretes mucous and contains PAS positive material.
The main diagnostic problem
in a benign choroid plexus papilloma is its distinction from a
papillary ependymoma.
The
vascular connective tissue stroma, the layer of columnar epithelium
and the absence of cilia characterize the former.
Atypical choroid papillomas
are recognized, with intermediate histology between papilloma and
carcinoma. They generally have a higher proliferation of epithelial
cells and increased mitotic activity, although clear diagnostic
criteria are lacking.
|
|
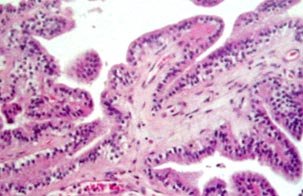
|
|
Ch.plexus pappiloma (H&E):
delicate papillary formations, each made up of a layer of
columnar or cuboidal epithelium resting upon a tenuous vascular
connective tissue stroma.
|
|
Malignant evolution may occur, with an incidence of
10-30%. The lateral ventricles are the most common sites for
malignant degeneration. With a clinical and histologic pattern of
malignancy, which is characterized by invasion, mitotic figures,
nuclear pleomorphism, necrosis, and
metastasis, these tumors are classified as carcinomas. The
carcinomas display an infiltrative pattern with features of
malignancy. In contrast, papillomas are
more homogenous.
As with most CNS neoplasms, confinement to the intracranial cavity is
usual.
While the choroid plexus
epithelium originates from the primitive medullary epithelium and
therefore is related embryologically to ependyma in both structure and function, it is
very different from the ependymal cells. It is more closely
akin, morphologically, to the surface epithelia that from the mucosal
linings in the other parts of the body.
Seeding can occur throughout the cerebrospinal axis;
this usually results in a solitary metastasis from a lateral
ventricular tumor in a child and in subarachnoid seeding to the spine
from a fourth ventricular lesion in an adult.
On rare occasions, widespread metastases from benign papillomas are observed.
A few cases of papillomas of an nonventricular origin
are reported. These are possibly explained by an origin in the
embryonic rests of choroid plexus.
Clinical
features:
Reports suggest that the median duration of symptoms was
1 month, and approximately one third of patients presented within 2
weeks. The tumor's presence often is heralded by nonspecific signs
and symptoms of increased intracranial pressure, which is present in
91% of patients, frequently in association with hydrocephalus due to CSF pathway obstruction, CSF over
production, CSF malabsorption due to hemorrhage or deposition of
proteinaceous tumor material into the CSF.
Vomiting is the most common sign in children. The
presentation can also include hemiparesis, homonymous visual field
defects, and generalized tonic/clonic and
focal seizures.
When the neoplasm arises within the cerebellopontine
angle, the presentation usually involves ataxia and cranial nerve
palsy, most commonly that of cranial nerves V, VII, or VIII.
In adults, headache is the most common presenting
symptom; this finding may be related to an alteration in head
position.
Sudden death can occur in
the third ventriclar tumor, causing acute
ventricular obstruction. Spontaneous hemorrhage may be the
presenting symptom occasionally.
The disease
burden can be significant, especially in young children. Morbidity is
associated with developmental delay in 39% of pediatric patients,
severe behavioral problems in 17%, and epilepsy in 48%.
Imaging:
|
On CT scan the
typical choroid plexus papilloma appears as a well marginated, smooth or lobulated iso- or high density mass protruding into the
lumen of the ventricle with strong contrast enhancement. This
marked homogeneous enhancement is related to the highly vascular
nature of the tumor. Tumoral
calcifications are uncommon in the pediatric age group.
The
MRI characteristics are of hypo to isodensity
on T1- and intermediate or increased signal intensity on
T2-weighted images. There are areas of internal signal void, predominantly
curvilinear, indicating enlarged intratumoral
vessels. MRI is superior to CT in assessing intraventricular
location and extension of the tumor, because of its
multidirectional imaging capabilities. Associated
findings include hydrocephalus, which may involve the lateral,
third, and fourth ventricles to varying degrees.
|
|
Invasion of adjacent neural
tissues, and irregular margins suggest carcinoma.
Differential diagnosis
include papillary ependymoma, metastasis,
meningioma, and pilocytic astrocytoma.
If symptoms
suggest, dissemination into the spinal canal should be considered and
neuroaxis imaging should be carried out.
At angiography,
enlargement of choroid arteries and multiple small tortuous vessels
in the arterial phase are characteristic. In the capillary and venous
phases, strong homogeneous accumulation of contrast medium in the
tumor is seen.
Management:
As a result of their benign nature and slow growth, CPPs
are amenable to complete surgical excision, with an
expectation of total cure. Not surprisingly, a favorable long-term
outcome is expected; the goals are a cure for all children and no
requirement for adjuvant therapy.
Despite advances in modern surgical techniques, such as
the use of surgical microscopes, bipolar coagulation, stereotaxy, and image-guidance techniques, a
significant risk of mortality and morbidity may be associated with
surgical treatment.
Extreme tumor vascularity, which is often present, may
hinder complete resection.
The perioperative management of hydrocephalus, which is
common in patients with CPP, is controversial; subdural fluid
collections, frequently caused by the persistence of a ventriculosubdural fistula, are often found in
the postoperative period; occasionally, these can cause symptoms of
increased intracranial pressure.
Increasingly widespread use
of endoscopic surgery may alter the future therapy of choroid plexus
neoplasms.
Radiation in children
is controversial. Careful follow ups in children with choroid plexus
papilloma was suggested by some in the past. It is now generally
agreed that surgical resection is the best option.
Radiation therapy
after surgical intervention usually is reserved for the treatment of
choroid plexus carcinoma.
Chemotherapy
has no reported benefit.
Prognosis:
Reports suggest that the 5-year survival rate was 100%,
and that tumors do not recur in half of the patients who underwent
subtotal resection. If the tumor evolves into malignancy, the
prognosis is dismal, with a 5-year survival rate of 26%. However, the
histologic appearance may not be predictive of biologic behavior
because some highly anaplastic choroid plexus tumors can be
clinically benign, whereas some histologically inactive tumors are
invasive.
The presence of mitotic figures, although rare in CPP,
may be predictive of the likelihood of both recurrence and malignant evolution.
Such histologic findings in the surgical specimen should result in
close clinical follow-up care of patients, especially in those whose
postoperative images show findings of residual tumor.
The
evaluation of atypical papillomas, or of
more widespread, benign appearing papillomas,
may be aided by evaluation of the proliferation index or by presence
or absence of various tumor markers. Patients with choroids plexus papillomas with a higher proliferation index or
the presence of certain markers have been shown to have worse
outcomes, generally observed as tumor recurrence.
|