|
Hydrocephalus is a condition in which
there is accumulation of CSF in the cerebrum due to a disturbance of
formation, flow or absorption of CSF. It is a pathological condition
rather than a specific disease.
Hydrocephalus occurs as an isolated congenital disorder in
approximately 0.9-1.5/1000 live births.
Hydrocephalus in
association with spinal dysraphism varies from 1.3 to 2.9/1000.
Dandy introduced the widely used and
generalized terms communicating (due to a blockage outside the
ventricles) and non-communicating (due to a blockage within the
ventricles) hydrocephalus. Both are, in essence, obstructive,
although at different sites. It may be acute or chronic,
depending on time course.
Arrested hydrocephalus
is an asymptomatic condition with non-progressive ventriculomegaly.
Compensated hydrocephalus
is a symptomatic condition, with non progressive hydrocephalus.
Normal pressure hydrocephalus
is a misnomer. It describes a condition in older adults of intermittently
raised ICP.
Non obstructive hydrocephalus
is a term used to describe enlarged CSF spaces due to loss of brain- external
hydrocephalus.
PATHOPHYSIOLOGY:
CSF production and absorption are in
dynamic equilibrium, with average production equaling average absorption
under normal physiologic conditions. CSF volume is approximately 150cc in
adults and is produced at a rate of 14-36 cc/hour. 50%-80% of the
CSF is derived from Choroid plexus. The remaining CSF is from cerebral
parenchyma from the capillary endothelium.
Choroidal CSF is formed as an
ultrafiltrate from the capillaries in the center of each villus. The
ultrafiltrate is then processed by the choroidal epithelium and secreted
by diffusion into the ventricles.
CSF circulates from the lateral
ventricles to the third ventricle through the foramen of Monro and then
to the fourth ventricle through the aqueduct of Sylvius. CSF then passes
through the foramina of the fourth ventricle into the subarchnoid spaces,
where it circulates to the primary site of absorption, the arachnoid
granulations of the sagittal and transverse sinuses. The emissary veins
of the dura and the lymphatic drainage system of the skull are other
sites of CSF absorption.
For a constant CSF volume to be
maintained, an equal volume of CSF must be reabsorbed by the arachnoid
granulations. If the absorption fails, ventricles enlarge at the expense
of the brain parenchyma, initially the immediate adjacent white or grey
matter rather than the cortex.
Continued enlargement disrupts the
ventricular lining and then the underlying white matter. There is an
increase in its water content due to transependymal flow of CSF from
elevated intraventricular pressure and the edematous parenchyma i becomes
spongy. Axonal and myelin destruction can occur with this increase in
extracellular water content. Ventricular diverticula may develop.
Interhemispheric fissure becomes elongated and thinned out.
Expansion of the skull in infants and
thinning and atrophy of the brain are resultant compensatory mechanisms.
In addition, there is contraction of the cerebral blood volume, and
alteration cerebrovascular circulation. CSF circulation is also altered.
Changes in Cerebrovascular
circulation: Earliest
change is the increase in cerebral venous pressure secondary to
compression of the unsupported cerebral veins. Cerebral arteries are
narrowed in chronic cases. Cerebral circulation time is prolonged. The
blood flow in the white matter, especially around the frontal and
occipital horns (prefrontal, parietal and visual association areas), is
selectively impaired and the same improves after shunt surgery.
Changes in CSF circulation: In non
communicating hydrocephalus, the subarachnoid CSF tends to flow normally
towards the cerebral convexities, as it is not dependant on choroid
plexus pulsations. In communicating variety, the flow is reversed back
into the ventricles.
Hydrocephalus
almost always results from an obstruction
(mechanical blockage or poor absorption) in the CSF pathways and only
rarely from overproduction of CSF, as in choroid plexus
papilloma and meningitis.
Poor absorption may
be due to defective archnoidal villi or rarely, to raised
venous pressure as in sinus thrombosis.
CLINICAL
FEATURES:
In acute cases, the patient is ill and
drowsy, and irritable, with headache, vomiting.
Infants with chronic hydrocephalus,
present with poor feeding, vomiting, reduced activity, and drowsiness.
There may be endocrine abnormalities due to prolonged pituitary
compression.
|
Examination reveals head
enlargement, dilated scalp veins, tense fontanelle, failure of upward
gaze, and 'sunset' sign; 'cracked pot' resonance of the skull
(Macewen's sign) may be detected in older children. Papilledema and 6th
nerve paresis may be Normal head circumference at birth is 33 to 36cm.
During the first year, it increases 2cm/month during the first 3
months; detected. 1cm/month from 4-6 months, and 0.5cm/month from 7 to
12 months. A diagnosis of hydrocephalus is indicated by circumference
increases across centile curves than by circumferences that are above,
but parallel to the 95%
|
|
Age
|
Head
circumference (CM)
|
|
At birth
|
35
|
|
3 months
|
40
|
|
9 months
|
45
|
|
4 years
|
50
|
|
|
Approximate standard head
circumference in boys
|
|
Head circumference of girls
older than 3 months is 3cm smaller than that of boys.
|
|
centile.
Chorioretinitis in a child with
hydrocephalus indicates an in utero infection with cytomegalovirus or
toxoplasmosios.
In chronic cases, adults, usually
complain of gait disturbances, memory loss, slowness of thought and
action, and urinary incontinence. Papilledema and 6th nerve paresis may
be the only clinical findings
Hydrocephalus in children:
Congenital hydrocephalus:
Hydrocephalus
is commonly a congenital disorder that can occur as an isolated finding
or as part of a complex congenital malformation syndrome. Congenital or
primary hydrocephalus, with an incidence of 1 in 1,000 births, is usually
a sporadic condition, but families with X-linked and autosomal recessive
patterns of inheritance have been reported. The X-linked form is more
common and is estimated to occur in 1 in 30,000 male births. This condition accounts for an estimated 25
per cent of male hydrocephalus not associated with myelomeningocele.
Hydrocephalus that follows an autosomal recessive pattern has been
described much less frequently.
Aquedect
stenosis
is the most common cause of hydrocephalus in the new born and may present
at a later age as well. The aquedect may be congenitally stenosed or
forked, having multiple blind outpouchings without patency. In addition,
aquedectal gliosis secondary to an ingrowth of fibrillary glia and
aquedectal stenisis secondary septum has been described. Aquedctal stenosis
can also be inherited in the rare Bickers-Adams syndrome (X linked
hydrocephalus).
Chiari malformation is frequently
associated with aquedect stenosis, probably due to compression, dorasal
displacement, and angulation of the aquedect.
|
About 80% of Myelomeningoceles
and encephaloceles also associated with hydrocephalus.
Approximately, 80% of the
patients with Dandy
walker malformation develop hydrocephalus in the first three
months of life. Hydrocephalus in Dandy walker malformation is,
probably, due to communication between the cyst and the subarchnoid
space. Symptoms may develop during the adolescent years.
Congenital toxoplasmosis, viral
infections, and cytomegalovirus can cause archnoiditis with subsequent
hydrocephalus.
Other rare chromosome
abnormalities are associated with malformations, such as, agenesis
of arachnoid granulations, and foramen of Monro and hydrocephalus.
Hydrocephalus in preterm
infants:
|
|
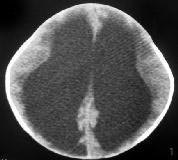
|
|
Congenital hydrocephlus-CT
|
|
Intraventricular hemorrhage(IVH) is the
most common serious neurological complication of the premature infant.
35-70% of the preterm underwieght infants sustain IVH.
The hemorrhage develops from the
germinal matrix capillaries that have not fully developed and are readily
susceptible to damage. The flow distribution to the germinal matrix leads
to a disproportionate cerebral blood flow in the periventricular
circulation during the period of greatest susceptibility. The caudate
nucleus and the cerebral cortex have high flow; the subjacent germinal
matrix has low flow. Hypotension followed by rapid volume reexpansion is
frequently the clinical context for hemorrhage.
The hemorrhage usually occurs within 48
hours of birth and occurs in the first 24 hours in 50% of cases. A later
onset is not uncommon.
Post hemorrhagic hydrocephalus usually
occurs in the first to the third week after hemorrhage. 20-50% of them
develop hydrocephalus, either transient or progressive and about 50% of
them may require surgical intervention.
Postnatal hydrocephalus:
|
Approximately 20% of cases of
hydrocephalus in children are related to a mass lesion, mostly
posterior fossa tumors. Tumors in the third ventricular region
(craniopharyngioma, intraventricular cysts, hypothalamic gliomas) can
also cause hydrocephalus.
Vein of Galen malformation can
compress the aquedect and posterior third ventricle and cause
hydrocephalus. Other aneurysms and AVMs have been associated with
obstructive hydrocephalus depending on their location.
Various toxins viral
infections, and nutritional deficiencies have been implicated in the
development of hydrocephalus. Vit A, and B12 deficiency, folic acid
deficiency, azo dyes, lysergic acid diethylamide mescaline,
triamicinolone acetamide, irradiation, and methyl mercury have all been
implicated .
|
|
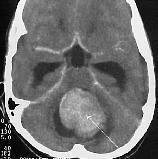
|
|
Hydrocephalus due to 4th
vent.meduloblastoma-CT
|
|
Benign communicating
hydrocephalus:
It is also known as Idiopathic External hydrocephalus.
It is characterized by rapid head growth, enlarged subarchnoid spaces
with little or no ventriculomegaly. The pathogenesis is not clear.
Cephalomegaly above 90th percentile with
normal neurological examination are the principle features.
CT and MRI reveal bilateral
extracerebral fluid collections, prominent sulci, normal ventricles, and
no evidence of compression of the brain. Chronic subdural hematomas must
be ruled out.
The natural history is one of gradual
resolution of the fluid collection. The family may be advised to avoid
prolonged supine positioning.
Hydrocephalus in adults:
Aquedect
stenosis, a developmental anomaly, may present in adulthood as well.
Hydrocephalus in
adults are more commonly due to obstruction due to intracranial mass
lesion or due to post infection, SAH, or trauma. They are discussed in
appropriate sections. History of any intracranial procedure may be cause
in some.
Normal pressure
hydrocephalus is discussed elsewhere.
INVESTIGATIONS:
Skull radiographs reveal sutural separation. Periventricular
calcifications in infants indicate in utero cytomegalovirus infection and
disseminated calcifications indicate toxoplasmosis. The inion is
typically low in children with aquedect stenisis, and high in
Dandy-walker malformation. In older children, there may be copper beaten
appearence or sellar enlargement which are nonspecific.
Cranial ultrasonography through the anterior fontanelle in
infants can be particularly useful for serial evaluations after
intraventricular hemorrhage. It also demonstrates ventricular morphology,
and masses.
CT and MRI clearly demonstrate the hydrocephalus and the
associated pathologies. The findings that favor hydrocephalus include
dilatation of the temporal horns, enlargement of the anterior or
posterior recesses of the third ventricle, narrowing of the
mamillopontine distance, narrowing of the ventricular angle, widening of
the frontal horn radius, and effacement of the cortical sulci. The most
reliable parameter is the dilatation of the temporal horns.
Isotope studies and ICP monitoring helps to identify
ventriculomegaly due to cerebral atrophy.
MANAGEMENT:
Medical treatment has
been largely unsuccessful, at least in chronic and progressive cases.
Diuretics may help in acute transient cases. Carbonic anhydrase inhibitor
has been claimed to reduce CSF production. Adrenocorticosteroids may
diminish the CSF flow.
Ventricular
shunting:
In transient
hydrocephalus, and in patients with high CSF protein or when CSF
infection is suspected external ventricular drainage is usually the
first line of treatment. External drainage introperatively helps prior to
certain tumor removal. Normally, the drainage is not maintained for
longer than two weeks to avoid infection. Studies suggest that, if the
drainage tube is tunneled well away from the insertion site, the
infection rate is low.
Established and
progressive hydrocephalus is treated with shunt insertion. Newborns with
hydrocephalus with cerebral mantle less than<2cm, should be treated
within 5 months to maximize the mantle thickness, associated with normal
IQ.
The shunts
drain CSF from the ventricles to a site of of superior absorptive capacity.
Many sites, including vessels, peritoneum, gall bladder, urinary bladder
have been used. Peritoneum has been proven to have the best absorptive
capacity. Ventriculo peritoneal, and ventriculo atrial shunts are
widely employed.
Implantable
shunts are composed of a silicone elastomer and are often impregnated
with barium. The shunts consists of a proximal catheter, a valve, and
distal tubing. Evolution of shunts has been guided by the need to reduce
the incidence of complications, some of which are trivial and
self-limiting whereas others are occasionally fatal. Financial
aspects are also important, as an episode of shunt infection can be
extremely expensive. Lately, antibiotic impreganated shunts are being
marketed.
There is no
perfect shunt. In general, there are three types of
valves:
1) A pressure
regulating valve opens at a preset pressure and maintains its pressure
across the valve, regardless of flow rate.
Slit valves, e.g..
Hotler,Upadyaya, Chabra: flow decreases gradually as differential pressure
decreases until closing pressure of valve (low pressure - 2-5cm water,
medium pressure - 5-10cm water, high pressure - 10-15cm water) is
reached.
Diaphragm valves, e.g.:
Pudenz, Ceredrain: flow remains roughly constant until closing pressure
is reached.
Ball valves, e.g.:
Hakim: similar pattern to diaphragm valves.
Programmable valves allow
the closing pressure to be adjusted externally using magnet.
2) Flow
regulating valve maintains a constant flow at different pressures to
overcome the complications of over drainage. The flow is regulated by
increasing valve resistance as pressure increases.
3) Siphon
resistant valves act by increasing the opening pressure of the system in
direct proportion to the vertical distance between the proximal and
distal ends of the distal tubing. This allows correction of hydrostatic
pressure, which changes when the patient changes his position. Positive
ICP is maintained, despite the position of the patient.
Insertion of a shunt must be regarded as
a lifetime commitment from the surgeon to the patient. meticulous
measures should be taken owing to the high frequency of potential
complications. Small skin incisions to avoid contact with skin, small
bony opening to prevent egress of CSF are recommended. The ideal position
for the ventricular catheter is in the frontal horn or in the occipital
horn to catheter blockage due to choroid plexus. The peritoneal catheter
should be positioned over the liver in the retrohepatic space to avoid
distal occlusion by omental fat.
Complications of shunts:
A baby with a new shunt would be
expected on average to undergo 2 shunt revisions for blockage during
first 10 years. Approximately 30% of infants with newly inserted shunts
will have a shunt complication within the first year.
The common shunt complications are,
obstruction, over drainage, and infection, and less commonly, seizures.
Obstruction:
A shunt can occlude at any site, but the
most common site is the ventricular end, usually by the choroid plexus.
The distal end can become occluded with
fat and with an abdominal pseudocyst. Precise endoscopic placement of the
ventricular end in the frontal horn to avoid the choroid plexus and
abdominal end at the retrohepatic space may minimize the chance of
obstruction.
RBCs, tumor cells, high protein level in
CSF have also been the cause of proximal tube obstruction. Body growth,
adhesions (associated with low-grade infection), and pregnancy may the
cause in distal end.
A shunt can occlude at any site,
but the most common site is the ventricular end, usually by the choroid
plexus. The distal end can become occluded with fat and with an abdominal
pseudocyst. Precise endoscopic placement of the ventricular end in the
frontal horn to avoid the choroid plexus and abdominal end at the
retrohepatic space may minimize the chance of obstruction. Body growth,
adhesions (associated with low-grade infection), and pregnancy may the
cause in distal end obstruction.
Patient presents with features of raised
ICT, and CT reveals ventriculomegaly.
If there is no spontaneous CSF flow
through a needle inserted into the shunt reservoir, the proximal is the
one that is blocked.
A shunt-o-gram, by injecting a
radioisotope into the reservoir and imaging both ends, may help to detect
the blocked end. ICP monitoring and CSF infusion studies will document
more precisely the shunt’s hydrodynamic properties.
The blocked end may be revised.
Alternatively, a new shunt system is established on the opposite side.
Overdrainage:
When there is excessive drainage, the
ventricles may collapse around the shunt, as in the 'slit ventricle'
syndrome. Siphoning effect of the shunt (hydrostatic pressure-25-75 cm
CSF) caused by column of CSF within peritoneal or atrial catheter sucks
fluid out of ventricles in upright position. Differential valve, even
high pressure 15 cm CSF, may not stop siphoning.
'Slit ventricle' syndrome: Some patients
will develop decreased transependymal flow and decreased intracranial
compliance. When a shunt malfunction occurs in these patients, the
ventricles fail to expand. Chronic low pressure within the ventricle and
intermittent shunt malfunction due to ventricular catheter abutting
against ventricular wall have been blamed
Clinical features include attacks of
headaches, vomiting, drowsiness and pallor associated with slit-like
ventricles on CT scan.
It is a difficult problem to treat.
Revision of shunt with a Siphon resistant valve, if available or a high
pressure valve may help. Occasionally subtemporal decompression or other
cranial expansion technique is needed.
Subdural Hematoma: Many
post-operative sub dural collection are asymptomatic and do not require
treatment. Others may cause reduced conscious level and focal
deficit, and require evacuation, and at times shunt removal and reinsertion
of shunt with a Siphon resistant valve, if available or a high pressure
valve as a second stage procedure.
Recurrent symptomatic sub dural
collections may need sub duro-peritoneal shunt.
Infections:
3-20% of the patients develop shunt
infection, and have increased mortality and increased seizures and may
affect long-term outcome in children. Staphylococcus epidermidis is
the causative bacteria in two thirds of shunt infections. Staph.
aureus and gram negative bacilli are also common. In neonates,
Escherichia coli and Streptococcus hemolyticus predominate.
Presenting symptoms include nausea,
vomiting, fever, lethargy, anorexia, irritability, and abdominal pain. In
addition, shunt may get blocked with features of raised ICT. CSF cultures
may be negative in 40% and one must have a high level of suspicion. On
rare occasions, 'shunt nephritis' may develop, secondary to
chronic low level infection of a shunt with subsequent immune complex
deposition into renal glomeruli.
Strict aseptic precautions, and
avoidance of shunt surgery in the presence of CSF leak or
intercurrent infection prevention.
In most cases of
shunt infection, the shunt may need to be removed. External ventricular
drainage may be required. Appropriate antibiotic is mandatory.
Intravenous Vancomycin is used widely while awaiting bacteriological
studies. Intrathecal/reservoir antibiotics do not provide additional
benefit. When the CSF culture is sterile for 3 consecutive days, it
is recommended that the antibiotics may be continued for about 10 days
and a fresh shunt is inserted
Some centers are
successful in treating shunt infections in situ without removing the
shunt.
Other rare
complications:
Secondary
sagittal synostosis may occur as a result of shunting in infants,
particularly premature babies with severe post-hemorrhagic hydrocephalus.
Miscellaneous complications of V-P
shunts (Operative misplacement of the shunt tubing, erosion of shunt
tubing through wall of abdominal organs, disconnections) occur
sporadically.
Miscellaneous complications of V-A
shunts (cardiac arrhythmias, cardiac tamponade, mural thrombus and
pulmonary emboli, detachment of distal catheter during shunt revision)
are uncommon, but more life-threatening than those in V-P shunts.
Endoscopic third ventriculostomy:
Internal decompression, by 'by passing'
the obstruction, restores normal CSF flow. If the obstruction is in the
ventricles or at the outlets of the third or fourth ventricles, internal
decompression may be possible; third ventricle should not be small. The
decrease in ventriculomegaly, is almost always less than that achieved
after a V-P shunt.
Lately, internal decompression by
rerouting CSF flow through the floor of the third ventricle using
neuroendoscopic techniques has
become dramatically refined.Third
ventriculostomy is claimed to be an effective alternative to shunt in
experienced hands.
OUTCOME:
20% of untreated children survive to
adulthood. Severely damaged babies with hydrocephalus are best treated by
shunting to prevent excessive head growth and its associated nursing
problems. 70% of babies with treated non tumoral hydrocephalus would be
expected to attend a normal school, with a normal IQ. Most become shunt
dependent and remain so for the rest of their lives.
About 50% of children with communicating
hydrocephalus may retain the potential to be independent of shunt.
|