|
Spinal AVMs are localized collections of blood vessels,
often abnormal in structure and number, representing an anomaly of the
spinal circulatory system with altered haemodynamics.
Epidemiology:
They are rare with an incidence of 3.3 to11% of spinal SOLs
with a male preponderance.
They usually present in the 4th or 5th
decade. Usually they extend over 4 or 5 segments and as a rule they are
located posterior or posterolateral in the caudal spinal canal.
Dural AVMs have preference for the thoracic and
thoracolumbar areas.
Classification and pathophysiology:
There are two main types:
A) Dural AVMs:
The nidus is embedded in the dural sheath of the nerve root.
They do not interfere with the blood supply of the spinal
cord and hence, believed to be acquired anomalies. They are low flow
shunts in the proximal dura of the nerve root, the adjacent spinal dura
or both.
It is supplied by a dural artery and empties into the
coronal venous plexus on the surface of the spinal cord leading to venous
hypertension and serpentine transformation of the coronal venous plexus.
As the medullary arterial supply is different arterial steal
is uncommon. Spinal cord ischaemia, cell loss and cord atrophy are due to
impaired arterial perfusion pressure as a result of venous hypertension.
They are predominantly found in the posterior part of the
lower thoracic cord and the conus.
B) Intradural AVMs:
Here the nidus is within the piamater or the spinal cord.
The current concept is, they are the result of maldevelpment
during the second stage of vascular formation (around the 6th
week of gestation), leading to persistence of thin walled tortuous
vessels with defective tunica media and elastica, primitive capillary and
precapillary channels and abnormal arteriovenous shunts. They are
frequently associated with other congenital anomalies.
They are further sub-classified into
a)
Intramedullary juvenile, and glomus types.
b)
Perimedullary (direct) AV fistulas
c) Cavernous
angiomas (intramedullary & extramedullary)
The juvenile type has a large intra medullary nidus
and contains cord tissue within its interstices. They often occupy the
entire cord at the involved level. Multiple medullary branches of the
anterior and posterior spinal arteries supply them. They are high flow
lesions and often a bruit may be heard at the level of the lesion.
They occur in adolescents and the young and have a poor
prognosis.
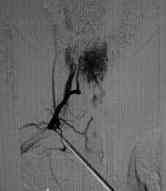
The glomus type is a tightly packed mass of blood
vessels, supplied by branches of the anterior spinal artery. They are
typically located in the anterior half of the cord and are more common in
the cervical region. Clinical presentation may be similar to an intra
medullary SOL.
They occur equally in both sex and become symptomatic in
younger patients.
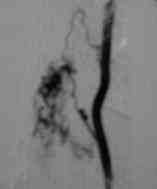
The perimedullary AV fistulas are direct
communications between spinal arteries and coronal venous plexus. They
are less common and found on the surface of the cord with no
intramedullary nidus. The flow is rapid with associated venous varices
and arterial aneurysms. Arterial steal and resultant cord ischaemia is
common. SAH is also a possibility. Usually they are located near the
conus and become symptomatic between the 3rd and 6th
decades.
They are further sub-divided into:
Type 1 – They are simple fistulas with by a
single feeder, often by the artery of Adamkiewicz.
Type 2 – They are of intermediate size with
more than one feeder, although one major feeder typically originates from
the anterior spinal artery. The draining veins are dilated with venous
ectasia at the site of the shunt.
Type 3 – They are giant and multipediculated
fistulas. The predominant feeder is from the anterior spinal artery and
the draining veins are greatly dilated and tortuous. Surgical excision is
usually not feasible.
Cavernomas occur in the vertebrae
(commonest), in the extradural space or within the cord substance and
represent 5 to 12% of all spinal AVMs. Myelopathy is due to small
haemorrhages and cord compression.
Clinical features:
Dural fistulas present with slowly
progressive myelopathy, usually involving the lower limbs as the lesion
is more commonly found in the thoracolumbar region. They are the
commonest and usually present in the 4th or 5th
decades.
Intradural AVMs frequently cause an
apoplectic event with intramedullary haematomas and subarchnoid
haemorrhage; upper limbs are also involved since the cervico thoracic
cord is commonly involved; they are found typically in younger patients
and uniformly distributed along the spinal cord.
There are no recent studies on the natural history of the
spinal AVM.
The patients demonstrate fluctuation of symptoms against a
background of steadily increasing disability; the majority become
disabled within three years after the onset of symptoms.
|
Pain is common and often multiradicular and an increase of
pain at nights and after hot bath has been reported; associated
anomalies may give a clue.
Pregnancy, menstruation, exercise and trauma are found to
precipitate or aggravate the symptoms; their significance remains
unclear.
Diagnosis:
Lately, MRI scanning and selective angiography are the
investigations of choice.
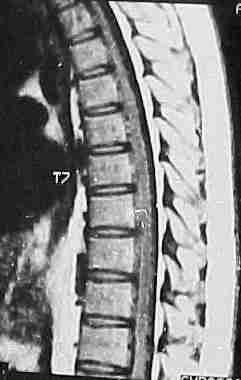
MRI scanning (T1) usually reveals a low
signal intensity in the cord; rapid flow may produce a signal or flow
void. T2 weighted images may show high intensity in the cord due to
cord swelling and may be useful in dural AVMs where T1 may be normal.
Cavernomas are diagnosed as well defined low intensity
areas with high intensity signals.
|
|
|
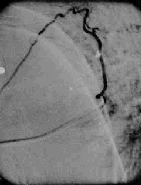
Selective angiography
is a must in every case where active treatment is contemplated.
|
large Dural AVM-MRI
|
|
|
|
|
|
|
|
|
|
Dural AVM-angio
|
Perimedullary
AVM-angio
|
Glomus type
AVM-angio
|
In the dural AVMs the nidus may be visualized at the
intervertebral foramen. Sluggish clearence of the contrast is a feature
of these lesions.
Myelography is still the choice in
patients with negative MRI as it often happens in small AVMs and the
dural AVMs. The accuracy with water-soluble contrast is about 90%. The
abnormal mass of sinuous turgid vessels as a ‘bag of worms’ is identifiable.
Management:
The aim is to eliminate the transmission of the venous
hypertension to the spinal cord and to suppress the arterial steal and
the risk of haemorrhage.
Active intervention is delayed to permit lysis and
absorption of the clot after an acute event.
The choice is between surgical excision and embolization and depends on the type of the AVM.
Surgical interruption of the dural AV fistula between
the nidus and the coronal venous plexus is preferred. Stripping of the
venous plexus may be harmful. The surgical procedure is simple and less
risky than embolization, which may accidentally aggravate venous
congestion.
|
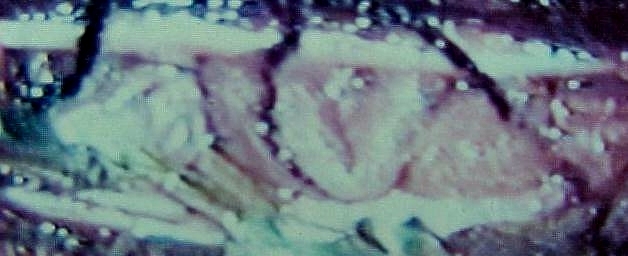
|

|
|
Dural
AVM-before interruption at surgery
|
Dural
AVM-after interruption at surgery
|
Total surgical excision is possible in glomus types
especially in the cervical region because of adequate collateral supply.
Embolization may be considered for the thoracic and lumbar lesions.
Surgery is not possible in the juvenile types and
embolization may be helpful.
Surgery is indicated perimedullary Type 1 fistula and
selected Type 2 fistulas. Embolization is difficult. Type 3 has poor
prognosis and embolization may help.
Presently, surgery is the only available option for cavernomas.
Prognosis:
The optimal time for treatment is before the disability is
substantial.
A short history (less than 3 months) implies good prognosis
for reversal of the deficit.
If the history is longer, minimal or temporary improvement
is often the result.
Long-term results of embolization are not yet known.
|