|
The
endovascular therapy or interventional neuroradiology plays an important
place in modern neurosurgery. In selected cases, it may lead to a total
and permanent cure per se, but in most cases, it will be an adjunctive
therapy to microsurgery or radiosurgery. With the development of
superselective angiography, and embolic materials, it has become a
rapidly growing sub-speciality of its own.
History:
Luessenhop
et al were the first
to describe embolizations of cerebral AVMs in 1969. He accessed the
internal carotid artery by the external carotid artery, after exposing it
surgically. Through the catheter silicon pellets of a defined diameter
were injected into the ICA. In line with the increased flow to the
pathologic vascular structures, these pellets followed the flow into the
malformation. Despite all the major insecurities inherent in this method,
it was practiced routinely at some places for many years. Newton
and Adams, Di Chiro and Ommaya did the
first embolisation for spinal angiomas. Serbinenko from
Moscow used the first detachable balloon for a CCF in 1974. Kerber
in 1975 used a calibrated leak balloon catheter (inflated with contrast
medium) to obstruct anterograde flow at a prescribed degree of dilatation
of the balloon, its contents were discharged into the artery distal to
the obstruction. Djindjian et al developed the technique of selective
catheterization of the branches of external carotid artery. Serbienko
succeeded in endovascularly accessing cerebral arteries by using
microballoons mounted on floating catheters � but this was limited to
unilocal vessel occlusions � comparable with the surgical ligations of
AVM feeding arteries. Kerber developed catheter tips
of varying wall thickness, achieving the effect of the calibrated leak
balloon by more forceful injection. Rosch et al described
embolizations with autologous blood clots. Porstmann et al presented
polyvinyl alcohol(PVA) particles of defined sizes as the material for
fine corpuscular embolizations. Sano et al presented freely
injecting polymerizing silicon into the ICA to cause deep embolization of
AVM nidi. Zanetti and Sherman used polymerizing
acrylate � developed and used as tissue sealant � for embolizations. Yakes
et al reported the safety and efficacy of absolute ethyl alcohol for
the embolization of vascular malformations fed by the ECA. Vinuela
et al used �Los Angeles cocktail� � two thirds contrast medium, one
third 95% alcohol, contains PVA particles and collagen material � this
caused better occlusion as the alcohol caused vascular wall
proliferation. Terada et al presented ethylene vinyl alcohol
copolymer in 1991.
The
introduction of Guglielmi detachable coils (GDCs) in 1991
has revolutionized the endovascular treatment of intracranial
aneurysms and is rapidly gaining popularity as an alternative
approach to surgical clipping in selected cases.
Requirements:
The success of endovascular procedures depends on the
catheter, guide wire and embolization material; technical equipment of
the endovascular angiography suite; pre-, intra-, and post-procedural
management of patients; and most importantly the experitise of the
physician.
Microcatheters require materials which increase
control of the catheter tip, improve movement and torque . The
proximal segment (thick wall, stiff) transmits both torque and
longitudinal movement almost without any deficit. The middle segment of
the catheter is more flexible and has a thinner wall but still features
high torque and control stability. The distal segment of the catheter is
characterized by a high degree of softness and reduced wall thickness.
Depending on the type of catheter used, the distal section is soft,
providing little control stability and increased flow catheter qualities
or has more torque stability and less flow dependability. Besides new
wall properties, the newest generations of microcatheters have
hydrophilic surface coatings, allowing for better performance.
Guide-wire
supported microcatheters permit
flow-independent movements of the catheter tip, making possible
advancement along fine vessels that branch off large-lumen main vessels,
such as perforating arteries that feed AVM�s. Guidewires feature
extremely floppy tips of different lengths that can be shaped at the tip,
which makes passing them along curved vascular formations easier. The
latest generation of wires is made from nickel-titanium and has a
hydrophilic surface coating to reduce friction between catheter and guidewire.
Seeker, QuickSilver, Sorcer and Terumo Glidewire are
some of the popular ones.
Embolization materials-The choice depends on the type
of vascular lesion, goal, and the vascular anatomy. None is ideal.
The following materials are in use.
Cyanoacylates can be injected through the
finest catheters because of their low viscosity and feature the best time
stablity of all embolizing media.
NBCA (N-Butyl-2-cyanoacrylate) hardens
immediately upon contacting free hydrogen ions of the blood and is mixed
with low-viscosity oily contrast media, or tantalum powder for
radiological visualization. It is liquid at the time of injection
but should solidify at the desired pathological target and should produce
an endovascular cast without migrating into the venous system. It has low
viscosity even when mixed with Lipiodol, can be injected through
microcatheters. Fluoroscopic monitoring is mandatary to see the
progress of material on injection.
PVA( Polyvinyl alcohol) particles of
defined sizes � 150-500micro m � may be used, but because of the limited
stability of the occlusion effect, they should be used only as
preoperative embolizing media.
Microcoils ( GDC )are available in
different lengths and with different helix diameters. They are advanced
through microcatheters and can occlude a high flow fistula or small
arteries. In contrary to previously available �free coils� modern coils
with electrolytic detachment mechanisms allow safe and precise placement
of the coil before detachment. Coils mounted with thrombogenic hairs or
additional glue injections may help to improve the results.
Silastic or latex balloons, Gelfoam in powder
form, Fibrin glue, Silicone spheres, Silk, and Ethibloc
are other agents occasionally used these days.
Radiography equipment- the preferential standard in radiography
equipment is biplane digital subtraction angiography with high-resolution
live roadmapping capability. Developments like rotational angiography
with the option of three-dimensional reconstruction may increase the
information on AVM architecture.
Anesthesia-Management
by a highly qualified specialist reduces risks. The necessity of stable
blood pressure is one reason to perform endovascular procedures under
general anesthesia. Another reason is the need for optimal imaging,
especially roadmapping.
Intra-procedural monitoring and tests-This
includes anesthesiologic monitoring and if available, neurophysiologic
monitoring, such as SSEP and EEG � and transcranial doppler .
Intra-operative functional monitoring by short acting barbiturates
(amobarbital sodium � 75-100mg) injected into microcatheters causes
transient deficits and tests the eloquence of the brain � but due to the
AVM, the amount of drug reaching the target brain is uncertain, a
negative test does not offer safety � the patient should be conscious and
co-operative.
Technique:
The
standard approach is by the trans-femoral route using Seldinger�s
technique. The guiding catheter(5F to 8F), which is located in the
carotid or vertebral artery is continuously flushed with heparinized
saline.
All
procedures are performed with the patients under general anesthesia
(propofol or halothane) and with systemic heparinization, including those
procedures that are performed to treat recently ruptured
aneurysms. A 5000-unit bolus of heparin and then 1000 units
of heparin per hour are administered until the end of the
procedure. The patient is reversed at the end of the procedure
with protamine sulfate (8-9 mg/1000 units of total
heparin administered) unless an embolic complication occurred.
Complications:
The complications most frequently reported in the
literature include rupture of AVM or aneurysm
either by the coil, microcatheter, or wire used to guide the
microcatheter, thromboembolic events, and thrombosis of the
parent artery ,accidental migration of embolic material to normal vessels
causing neurodeficits.
Cranial nerve palsies due to occlusion of the ECA branches
supplying the transcranial course of the cranial nerves is a possibility.
Scalp necrosis due to occlusion of branches of ECA, premature
balloon detachment, pulmonary embolism, and infection are other possible,
but rare complications.
Post-operative monitoring of vital parameters is necessary because
fluctuating blood pressure poses particular dangers as a result of the
changed hemodynamics of the brain. Multiple neurologic examinations and
neuropsychological tests are important follow-ups in addition to MR
imaging studies and angiograms.
Cerebral AVMs:
|
Curative embolization:
To achieve definite treatment the complete obliteration of
the AVM nidus must be the goal of intervention. Only small AVMs with
easily accessible feeders can be occluded in a permanent way without
increasing the risk of the procedure, so most of the reported
endovascular series have only a ew completely occluded AVMs. More than
one intervention is often necessary to achieve permanent, complete
obliteration of a nidus, because, occluding all compartments of a large
AVM would last too long and second the sudden reduction of large shunt
volumes might cause too much change in hemodynamic conditions. But
commonly, feeding branches for which a sufficiently selective catheter
position could not be achieved in a first or second session remain
un-occluded.
But commonly, feeding branches for whicha sufficiently
selective catheter position could not be achieved in a first or second
session remain un-occluded.
The stability of the occlusion is the second prerequisite
for a successful endovascular therapy of cerebral AVMs for which the
types of occlusion and the embolizing material used are considered.
The stability of the occlusion, that is the prevention of
the formation of collaterals into the nidus is best achieved if the
nidus is filled with the embolizing medium. For this, a correct
assessment of the flow condition in the respective compartment and
select the proper quantity and mixture ratio of glue and dye.
|
|
|

|
|
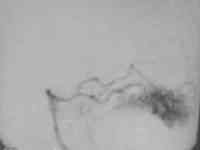
Occipital AVM
|
... post embolization
|
|
|
|
|
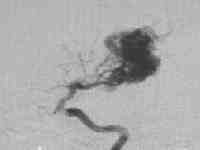
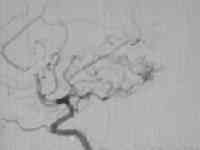
High flow temporal AVM
|
....post embolization residue
|
|
|
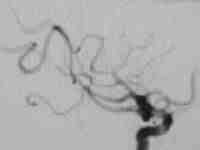
|
|
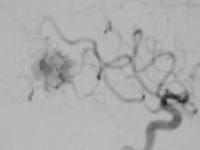
Parieto occipital AVM
|
...post embolization
|
|
|
The stability of glue depends on whether they present in
the AVM as isolated flocks with large thrombosed areas in between or
fill the nidus as a solid casting � the latter scenario is curative.
Intra-procedural and post-procedural angiogram permits conclusions as
to the stability of the occlusion.
Pre-operative embolization:
The goal of pre-operative embolization is turning the AVM
that was supposed to be inoperable into an operable AVM. The risk of
the procedure can be low, and the risk of the operation with regards to
blood loss is decreased. The goals of this form of Embolization
are reduction of the arteriovenous shunt volume; occlusion of
deep-feeding arteries; including perforating arteries and obliteration
of intra-nidal aneurysms or intra-nidal large AVFs.
NBCA is used because the nidal penetration is good.
Occlusion of feeding arteries with insufficient nidus obliteration
results in a �cured� post-embolization angiogram but quickly induces a
collateral supply, creating difficulties for surgical removal.
Depending on the size of the AVM, number of feeders and clinical
presentation, one or more pre-operative sessions may be necessary,
allowing a safe and much easier microsurgical removal with lower rates
of morbidity and mortality.
|
Pre-radiosurgical embolization:
The goal of pre-radiosurgical embolization is to make
radiosurgery feasible and to minimize the bleeding risk in the latency
period. The aim is to reduce the nidus diameter to a volume of less than
10ml, embolization of weak elements in the angio-architecture of the
nidus (eg: flow related or non flow related aneurysms, venous pouches or
high flow fistulas) and minimization of dural supply to the AVM nidus.
The occlusion must remain unchanged over time despite the fact that it is
only a partial occlusion. The resulting size and shape of the parts of
the AVM remaining open for radiosurgery is important � the more
irregular, the lower the feasibility of radiosurgery. In addition to size
and shape is the relationship to eloquent areas of the brain influencing
dosimetric planning.
Deep spherical AVM remnants are treatable by radiosurgery,
whereas large, spotted, superficial remnants in eloquent areas represent
an endovascular result untreatable by subsequent radiosurgery.
Palliative embolization:
Large and giant AVMs cause primary seizures, headache and
other focal symptoms. Surgery is not possible because of multiplicity of
feeders, complex angio-architecture & large size. For these patients
palliative embolization may be offered with goals of reducing the shunt
volume in the nidus to obtain seizure control or to reduce focal hypoxia.
Another goal is to embolize feeding artery aneurysms.
Care should be taken to prevent obliteration of venous
outflow. These patients should have regular clinical and angiographic
examinations.
DURAL AVMs/F ISTULAE:
|
Caroticocavernous
fistulas:
CCFs can be fast-flow (type A) and slow-flow (type B, C,
D).
Type A
is found in ruptured aneurysm and traumatic ones and ECA is not
involved; ICA contributes through small meningeal branches not usually
accessible to detachable balloons.
Inflatable balloon via the endarterial route is used,
reportedly with 80% success. In failed cases, the venous approach
through the inferior petrous sinus or the superior orbital vein (which
may have to be exposed surgically) may be tried. In some ICA has to be
sacrificed.
Type B
is a dural shunt/AVM, between meningeal branches of ICA and the
cavernous sinus;embolization is not possible usually.
|
|
|
|
|
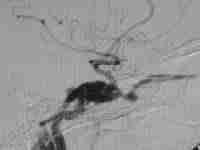
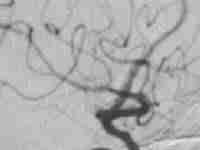
Type A CCF
|
.post embolization-ICA preserved
|
|
|
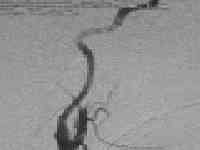
|
|
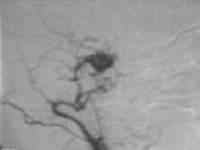
Type C CCF
|
...post embolization
|
|
Type C is
a dural shunt between meningeal branches of ECA and cavernous sinus;
successful embolization is possibleType D is dural shunt between
meningeal branches of ICA and ECA with cavernous sinus; embolization
through ECA feeders can be attempted. ICA feeders cannot be embolized
usually.
Type D is
dural shunt between meningeal branches of ICA and ECA with cavernous
sinus; embolization through ECA feeders can be attempted. ICA feeders
cannot be embolized usually.
In other
Dural fistulas cure is achieved in 50% with endarterial
embolization of the ECA Embolization with particles has low
morbidity rate but recanalization chances are high. Liquid materials have
high cure rates and high morbidity rate. Coils are not used.
Dural AVMs of the spinal cord are often treated
by selective embolization
Vein of Galen malformations are treated by transarterial or the
transvenous-transtorcular route.
The primary aim is total occlusion. Partial occlusion helps
in controlling the congestive cardiac failure in neonates till the
optimal time for definitive treatment.
CEREBRAL ANEURYSMS:
Surgical
clipping has been the accepted treatment of choice for intracranial
aneurysms during the last several decades. However, the
management of intracranial aneurysms has become controversial
with the recent advent of endovascular techniques.
The
endovascular treatment of intracranial aneurysms has evolved
rapidly. Initially, treatment of aneurysms using the
endovascular approach was limited to occlusion of the parent
vessel. Subsequently, detachable balloons were placed within
the sac of the aneurysm, preserving the parent artery, and
this technique is still used extensively in the Ukraine
by Victor Scheglov.
|
More
recently, Hilal in 1988, first reported aneurysm occlusions
with nonretrievable coils.
The
introduction of Guglielmi detachable coils (GDCs) has revolutionized
the endovascular treatment of intracranial aneurysms.
Indications:
Many
physicians consider surgical clipping to be the treatment of
choice, reserving endovascular therapy using GDCs for
aneurysms that cannot be treated surgically or for patients
who are considered to be nonsurgical candidates because of
the severity of their medical condition.
|
|
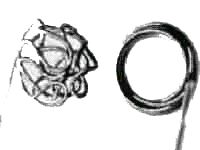
|
|
GDC Coil 3 D & Regular
|
|
Conversely, several physicians in Europe
have adopted an opposite position and consider the
endovascular approach with coils to be the treatment of first
choice, reserving surgical clipping for
those aneurysms for which coiling has failed or those that are
incompletely occluded after coiling.
Selection
criteria:
The
criteria for selecting patients to be treated using GDCs
are continually being redefined.
The
so called �unclippable � aneurysm is difficult for the
interventionist as well. The decision to treat using GDCs is
based on the presenting medical condition, location of the
aneurysm, and, in part, aneurysm shape.
Aneurysm
shape and size-
spherical aneurysms with small necks have a greater chance of
complete occlusion using GDCs than do large aneurysms with
broad necks of 5 mm or greater in diameter.
The dome-to-neck ratio is not the only measurement criterion
to be considered when treating aneurysms using GDCs. The
neck diameter will affect the morphological occlusion
outcome. Despite the favorable dome-to-neck ratio, the width
of the neck allows prolapse of the coil into the parent
artery, preventing complete occlusion.
|
Aneurysms with an
unfavorable dome-to-neck ratio and aneurysms with a
favorable dome-to-neck ratio but with wide necks are not
totally excluded from treatment using GDCs. These aneurysms
may be amenable to coiling with the adjunctive use of a
balloon positioned across the neck of the aneurysm, which
was coined the remodelling technique by
Moret. The remodelling technique can fail
secondary to vessel tortuousness, preventing placement of
the balloon, or because of limited balloon sizes and
available balloon inflation configurations, which prevent
successful complete occlusion of the aneurysm. Over inflation also
increases the risk of balloon failure, either by
rupture or by loss of the wire's ability to occlude the
distal lumen, preventing inflation. Continual improvements in technique, balloon design, and size availability
will provide improved outcomes. This technique shows promise
in the treatment of surgically difficult aneurysms and
aneurysms with unfavourable geometry using GDCs.
Aneurysm location- Configurations unfavourable
to successful coiling include locations where multiple branches
arise, such as the MCA trifurcation. The branching
vessels can obscure visualization of the aneurysm neck, increasing
the risk of coil protrusion into the parent artery or
adjacent branch vessel.
A second unfavourable configuration is that of a
branch vessel arising from the neck of the aneurysm, which
commonly occurs with PComA aneurysms. AComA aneurysms are
occasionally difficult to assess based on the pretherapeutic
angiogram regarding whether the geometry is suitable for
coiling.
|
|
|
|
|
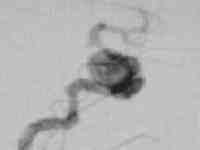
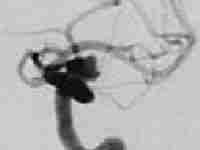
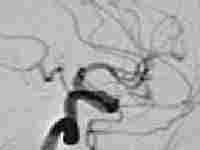
Giant intracavernous aneurysm
|
post balloon
occlusion of ICA
|
|
|
|
|
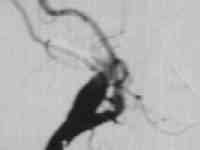
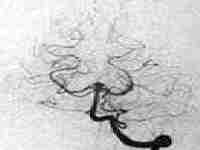
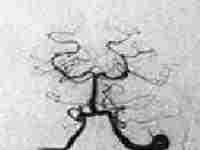
Ophthalmic aneurysm
|
...post coiling
|
|
|
|
|
Basilar tip aneurysm
|
...post coiling
|
|
Accurate
measurement of the aneurysm neck and relationship to the
parent artery of many aneurysms may be difficult to obtain
from the pretherapeutic angiogram. In these situations, subselective
injections with the microcatheter may provide better
assessment of the aneurysm geometry, or 3D-CT angiography
can define the aneurysm and in many cases, only placement of
the initial coil will provide the true measurement of the
aneurysm neck.
Technique:
The
technique is broadly the same as in AVMs.
The
choice of microcatheter is dependent on the aneurysm size,
with the Tracker 10 systems being used primarily for
aneurysms measuring 8 mm or less in diameter and the Tracker
18 systems being reserved for larger aneurysms. The guidewires
used with the microcatheters varies
GDC
coils are used more
commonly. GDCs are manufactured with two primary wire
diameters (GDC 10 and GDC 18), corresponding in size to 10
and 18 . The advantage of the smaller system is that the coil
itself is softer than that of the larger system and is of
similar secondary helical diameter, reducing the risk of
perforation. The primary disadvantage is the limitation in
size of the secondary helix to 10 mm, and aneurysms
larger than 1 cm need to be treated using the GDC 18 system,
which has secondary helical radii up to 18 mm. Therefore,
accurate measurements of the aneurysm size before coiling is
essential to select the appropriately sized coiling system.
The use of GDC 10 coils through a No. 18 microcatheter is not
recommended because the coils may buckle and become damaged.
The
development of GDC 10 and 18 soft coils further
reduced the risk of aneurysm perforation over the initial
standard GDC 10 and 18 coils. A second advantage of the soft
coil is reduced configurational memory of the secondary helix,
which allows the coil to better adapt to the remaining
space within a partially coiled aneurysm, improving the
ability to densely pack the aneurysm. Additionally, the
reduced configurational memory of the soft coil allows
improved results using the remodeling technique because the
coil has less tendency to revert to its original shape and
decreases the incidence of coil protrusion through the neck of
the aneurysm.
The
use of two-dimensional coils has also been found to
be an advantage in the treatment of aneurysms. Deployment of
the first loop of coil with a shorter radius into the
aneurysmal sac decreases the risk of perforation and migration
of the coil into the parent vessel.
Wide-necked
aneurysms having an unfavourable neck-to-fundus ratio are
difficult to embolize with conventional GDC coils without the
use of balloon remodelling or other supplemental
methods. A neck-to-fundus ratio close to 1.0 constitutes an aneurysm
difficult to treat by using the conventional GDC coil without
resorting to an adjunctive method. compared with smaller-necked
aneurysms.
The
technical difficulty encountered in providing stable support for
the conventional coil mass within a wide-necked aneurysm has
prompted the suggestion of various methods, in addition to
balloon remodelling, such as coiling through a stent or the use of
two-catheter techniques. Use of an inherently complex-shaped
GDC coil with a propensity to form a 3D cage spontaneously
after deployment. The complex shape results in a lower
tendency to prolapse into the parent vessel lumen. The 3D coil
seems to provide an inherently stable framework in the wide-necked
aneurysm, which enables the subsequent deployment of four
conventional GDC coils without the use of additional
microcatheters. During deployment, the 3D coil is stiffer
compared with the conventional or two-dimensional GDC variant.
This subjective stiffness is actually translated into greater
local mechanical stress being applied to the aneurysm during
3D coil placement. Although the eventual role of
this type of coil in aneurysm embolization will be defined
only with longer-term experience, the 3D-shape GDC seems to
provide a single-microcatheter solution for the endovascular
treatment of aneurysms harbouring a wide neck or an
unfavourable neck-to-fundus ratio.
When
the remodelling technique is performed, latex balloons and
silastic balloons may be used.
All
coils are deployed with live simultaneous biplane roadmapping.
Coiling
is performed until no further coils could be placed within the
aneurysm.
Heparin
is reversed with protamine sulfate, and the patient is woken
up from propofol sedation and is transferred to the Neuro Intensive Care
Unit for observation.
Patients
with unruptured aneurysms are usually discharged within 48
hours after uneventful coiling.
Patients
with acutely ruptured aneurysms are monitored in the Neuro
Intensive Care Unit. Endovascular balloon angioplasty and
papaverine infusions are performed as necessary for patients
who developed symptomatic vasospasm in some centres.
Follow-up
angiography, are performed at 6 months, 1 year, and 2 years
after treatment.
Post
procedural problems:
Ideally,
treated aneurysms should have dense and tight coil packing,
which requires experience and has been improved with the
development of the soft GDC.
The problem of residual neck & neck regrowth in
apparently completely occluded aneurysms after coiling using
GDCs remains a potential concern. One explanation provided by
the literature is that during coiling using GDCs, there is no
mechanical apposition of the aneurysm neck endothelium, allowing
for potential recanalization and regrowth. These neck
regrowths tend to be small, less than 5% of the original
aneurysm size, and can occur as early as 6 months or as late
as 2 years after the procedure.
The
inherent tortuousness of the cavernous region at times can
prevent stability of the microcatheter tip during placement of
the last few coils into the aneurysm neck. Movement of the
microcatheter during the final stages of the procedure can
result in ejection of the microcatheter tip from the aneurysm
neck, preventing complete packing, which results in neck
remnants and possible neck regrowth.
In
basilar tip aneurysms, microcatheter stability is usually less
of a problem; however, the relationship of the aneurysm neck
to the origins of the posterior cerebral arteries becomes the
limiting factor in preventing complete occlusion. Minimal coil
protrusion at this location can cause partial stenosis of the
PCA, resulting in the increased risk of thromboembolism or
thrombosis.
Incomplete occlusion is not the desired outcome, and often,
further treatment using the remodeling technique, parent vessel
occlusion, or surgery can be performed.
The
remaining incompletely occluded aneurysms should be followed, with
one of three possible outcomes: 1) progression to complete
occlusion, 2) the residual lumen remains stable, and 3) there
is enlargement of the residual lumen.
The
literature reports about a 2.2% subsequent haemorrhage rate
among ruptured aneurysms that are incompletely occluded with
coils. It has been suggested that partial occlusion of an
aneurysm treated in the acute phase protects the dome of
the aneurysm from subsequent bleeding and allows a second
treatment at a later date when the patient has clinically
recovered from the SAH.
Vasospasm occurs and is attributed to the
SAH. Increased incidence of symptomatic vasospasm in acutely
ruptured aneurysms treated using coils, because blood and
hemoglobin degradation products are not removed from the
subarachnoid space has not been reported.
Cerebral and vertebral tumors:
|
The aim is to devascularise the
tumor. This reduces the intraoperative blood loss, provides easier
manipulation of the tumor at surgery, and shortens the operation
time.
The
embolization is carried out ideally 12- 24 hours before the planned
definitive surgery to avoid the probable development of collateral
circulation and revascularization of the tumor.
Meningiomas, more commonly, are subjected to preoperative
embolization procedures .They arise from the cap cells of the
meninges and hence they are fed primarily by the
meningeal arteries. As they enlarge additional supply comes from
the pial vessels; peripheral portion may be supplied by cerebral
arteries.
The ultimate result is dependant on the percentage of
dural supply, the ability to reach the different arterial feeders and
the possibility to carry the embolization intratumorally. Hence
basal tumors are usually inaccessible.
|
|
|

|
|
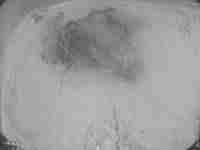
parietal meningioma
|
�post embolization
|
|

|
|
|
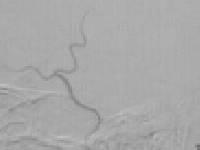
large skull base tumor
|
�post embolization
|
|
Surgery for Vertebral hemangiomas is made easy following
embolization.
Specific complications include skin necrosis by extensive
occlusion of the cutaneous branches of the ECA, and cranial nerve palsies
due to occlusion of the ECA branches supplying the transcranial course of
the cranial nerves.
Recanalization procedures:
It includes �local intra-arterial fibrinolysis � which is
mostly carried out in thromboembolic occlusion of the middle cerebral
artery and the basilar artery, and the � percutaneous transluminal
angioplasty in cases of ICA, the subclavian or vertebral artery.
Relatively new is the application of this procedure in the
management of vasospasm, which is still in the experimental stage.
|