|
By definition, it excludes traumatic and aneurismal
hematomas.
25% of all the strokes present with intracerebral haematoma
(ICH) and account for 2-4% of all deaths. They are twice as common as
SAH. Over two thirds are known to be fatal. The patients are usually
middle aged or over, with a male preponderance. The incidence is about 1
per 10,000 with a 30day mortality of 44%.
Etiology:
There are two categories, primary and secondary.
|
Primary ICH:
It is associated with hypertension and distinct from
haemorrhagic infarcts. It has been suggested that hypertensive changes
in the arterial wall, such as, hyaline degeneration, and microaneurysms
are at fault. Another suggestion is the thin walled vessels (such as
lenticulostriates), originating directly from the main vessel are
subjected to higher intravascular pressure than the cortical vessels
and tend to rupture.80% of them are supratentorial.
Mostly, the location is central and deep.
|
Putamen††††††††††††††††††††††††
-55%
Thalamus††††††††††††††††††††††
-10%
Subcortical whitemater† -15%
Cerebellar hemisphere†† -10%
Pons††††††††††††††††††††††††††††
-10%
|
Secondary ICH :
It is associated with a medical condition other than
hypertension, representing about 20% of all ICHs.
They may be due to:
Coagulopathies (10-15%)-Among these,
platelet disorders are important. About 5% of those receiving heparin,
irrespective of the dosage, develop thrombocytopenia. The platelet
defects may be hereditary (Von Willebrandís disease) or acquired through
drugs (Aspirin, penicillin, or new cephalosporins) or through disease
(myeloproliferative and dysplastic disorders, uraemia, cirrhosis, SLE,
multiple myeloma).
AVMs (7%) represent a heterogenous group
with different histological types (cavernoma, AVMs, venous angioma and
capillary telangiectosis).
Vasculopathies (5%), such as cerebral
amyloid angiopathy, polyarterites nodosa and necrotizing vasculopathy in
drug abusers, tend to produce multiple subcortical haematomas.
Tumors (2%) such as glioblastoma and
metastatic tumors such as, melanoma, choriocarcinoma, renal cell
carcinoma and bronchogenic carcinoma, are the most frequent tumors in
producing ICH.
Pathophysiology:
The hematomas may be massive (>5cm ) with extension into
the ventricles or may be small (<1.5 cm ).
The extravasated blood forms a roughly circular or oval mass
which grows in volume for a brief period. Adjacent brain tissue is
displaced and compressed resulting in extensive edema and ischemia.
Ischemic area may be much larger than the area of clot.
Cerebellar and brainstem ICH may produce obstructive
hydrocephalus which may add to the problems. In large hemorrhage, there
is midline shift and the vital centers are compromised.
Rebleeding is rare.
Resolving haematomas may develop into a cyst over a period
of months, with a gliotic wall which may be orange colored due to
haemosiderin laden macrophages.
Clinical features:
It depends on the site and size of the hematoma.
Sudden headache, vomiting with depressed level of
consciousness and focal signs is the usual mode of
presentation.
Absence of neck stiffness may help to exclude SAH .
The large ones are usually associated with LOC.
In putaminal ICH, the patient develops sudden
hemiplegia with conjugate horizontal gaze deviation towards the clot.
Speech may be involved if the dominant hemisphere is involved.
In thalamic ICH, the findings are as in putaminal
ICH; in addition, there may be neck retraction, paralysis of vertical
gaze with upward gaze palsy, inequality of pupils, and skew deviation
with the contra lateral eye being displaced downward and medially.
Cerebellar ICH presents with severe
headache, nausea and vomiting and imbalance and depressed level of
consciousness.
Pontine ICH present with coma, pin point pupils
and decerebrate rigidity.
Cortical ICH may present with headache and
seizures.
|
Investigations:
CT scan will reveal the clot and other
associated features such as midline shift and hydrocephalus. A contrast
CT may suggest a vascular problem, which may necessitate an
angiography.
MRI gives a better delineation of the
above; in addition
|
|
|
|
|
the age of the haematoma can be guessed. MRI may suggest
an associated AOVM.
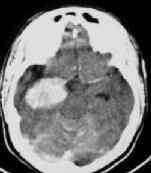
|
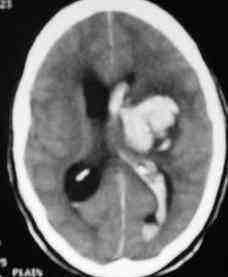
Thalamic hge. with
intraventricular extension
|
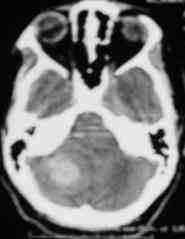
Cerebellar hge
due to Cavernoma
|
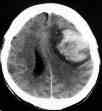
Ext.capsule Hge
|
|
Angiography should be carried out
whenever there is a suggestion of vascular malformation, in the absence
of previous hypertension or coagulopathies before a life saving clot evacuation.
When surgery is not planned, the angiography can wait for few weeks to
avoid a false negative angiography.
Coagulation studies must be done as a routine in addition
to ECG, chest X-ray and other general investigations.
|
|
|
Management:
|
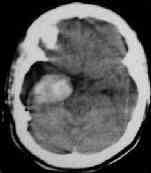
intratumoral
bleed ---- plain and contrast CT
|
Supportive
care control of hypertension, reduction of ICP without
compromising the CPP and prevention of complications are the
mainstay. Fluid and electrolytes and tissue oxygenation must be
closely monitored. The aim is to avoid secondary events.
An aggressive decrease of high BP may lead to cerebral
ischaemia. Ideally, it should not be lowered below 150mm Hg systolic and
100 mm of Hg diastolic.
Should general measures to control the raising ICP fail,
hyperventilation may help; but must be employed with careful watch on
pCo2, arterial blood pressure and preferably with ICP monitoring as well.
The CPP should not be compromised.
Osmotherapy with mannitol may help only when the serum
osmolality is lower than 300 mosm/kg.
Prophylactic anti convulsant therapy is advised by most
physicians with no supporting evidence.
The role of Surgical intervention is controversial. Neurosurgeons
and neurologists advocate that large cerebellar hemorrhages
with compression of the brain stem or obstruction of the
fourth ventricle should be surgically removed as soon as possible.
Surgical removal of large lobar hemorrhages in young patients
who are clinically deteriorating has also been recommended
based on anecdotal experience. On the other hand, the results of
such surgery in hematomas within the basal ganglia and other deep
structures are unacceptable. Standard craniotomy for surgical
removal of primary brain stem or thalamic hemorrhages has been
all but abandoned because of the extremely poor outcomes in
almost all patients.
Craniotomy:
Craniotomy
and evacuation of the clot has been the standard approach for removal of
intraparenchymal hemorrhage. In addition a decompressive
craniectomy with a duraplasty is prefered by some. Its
major advantage is adequate exposure to remove the clot. It is not
difficult or time-consuming. The major disadvantage of a more
extensive surgical approach is that it may lead to further
brain damage, particularly in patients with deep-seated hemorrhages.
In addition, the effectiveness of clot removal by craniotomy is
far from ideal.
There have been numerous nonrandomized
series comparing craniotomy and best medical treatment of ICH.
Recently Morgenstern and colleagues reported a single-center, randomized
trial (STICH Trial) of standard craniotomy versus best medical
therapy in patients with supratentorial
ICH; the goal was to perform surgery 12 hours after symptom onset.
Patients had to have a supratentorial ICH with a volume 10 cm3
and a GCS score of 5 to 15. Of the 34 patients in the randomized
trial, 17 were randomized to removal of the ICH by standard
craniotomy. The median time to surgery for the 17 patients was
8.3 hours (minimum 3.75 hours and maximum 26.1 hours). The 6-month
mortality for the surgical group was 17.6% compared with 23.5%
for the medical group. The median 6-month Barthel index score
for survivors in the surgical group was also similar to the
median Barthel index score for the medical group. However, the
groups were not balanced with regard to ICH location. Only 1
of the 17 patients (6%) in the surgical group had a lobar
hemorrhage compared with 7 of 17 patients (41%) of the medical
group.
Nonrandomized treatment series of
patients with cerebellar hemorrhage report good outcomes for
surgically treated patients who have large (>3 cm) cerebellar
hemorrhages or cerebellar hemorrhages with brain stem
compression or hydrocephalus. In these patients, medical
management alone often results in bad outcomes. Smaller cerebellar
hemorrhages without brain stem compression that are managed
medically do reasonably well.
Newer
techniques: The
grim results of conventional craniotomy have stimulated a search for more
tolerable, less traumatic, and safer methods of clot removal. Technical
advances in removal of ICH include improved localization of
the hemorrhage by stereotactic devices or intraoperative ultrasound
and better surgical techniques.
Innovations
in devices to break up and remove the blood clot include
modifications of an Archimedes screw inside a cannula, a specially
designed ultrasonic aspirator, a modified nucleotome, a double track
aspiration, and intraoperative CT monitoring. Intraoperative
ultrasound has also been used to identify the hemorrhage and
monitor its removal in real time..
Stereotactically
controlled endoscopic evacuation is gaining popularity. It
permits localization of the lesion, and removal of the clot is performed
under optic control, which may be important in cases of cryptic
arteriovenous malformations. This high-tech method may be simple, fast,
safe, and effective and provides for continuous intraoperative volume
removal.
Fibrinolysis aids rapid
dissolution of the remaining blood. The aim is to achieve a mass
reduction as well as to reduce the extension of perifocal edema and
minimize the amount of tissue damage. The most commonly used thrombolytic
protocol has been administration of 6000 U of urokinase once
or twice daily via a catheter into the bed of the hematoma
with subsequent drainage and aspiration. A urokinase washout
can be performed for up to 7 days after the bleeding.This procedure is
often repeated over several days until the majority of the
hematoma has been aspirated.
Hematoma
puncture and catheter placement for fibrinolytic therapy could be
achieved with high accuracy and safety using frameless stereotaxy. This
method, reportedly, allows unrestricted trajectory selection with
catheter positioning along the main hematoma axis. Further studies are
required to investigate if frameless stereotactic puncture and clot lysis
could contribute to improve the outcome of patients with ICH.
Outcome:
The
natural course of spontaneous ICH leads to a 30-day mortality rate of 45%.
The patient's initial level of consciousness, hemorrhage size, and
intraventricular extension of blood has proven to be accurate predictors
of outcome. Less commonly, age, sex, hypertension, and mass effect may
indicate harmful effects on outcome in patients with ICH.
The
author recommends that patients with smaller hematomas who are alert,
stable, or improving should be treated medically and the patients with
larger hematomas who show progressive neurological deficit, prolonged
functional impairment, and intracranial hypertension should be treated
surgically. Patients with a GCS score <4 should also be
treated medically because they uniformly die or have extremely
poor functional outcome that cannot be improved by surgery.
Easily accessible supratentorial hematomas with mass effect, especially
in the young and in those with a GCS score >5, must be evacuated. The
aim of surgery should be the removal of as much of the clot as possible,
with minimal disruption of surrounding brain tissue. If possible, surgery
should also remove the underlying cause of hemorrhage, such as
an arteriovenous malformation, and prevent complications of
ICH such as hydrocephalus and mass effect of the blood clot. More complete
clot removal may decrease elevated ICP and local pressure effects of
the blood clot on the surrounding brain. Stereotactic aspiration
may be associated with better outcomes than standard craniotomy;
but this hypothesis has yet to be tested in a randomized
study. Ultra-early removal of ICH by localized, minimally
invasive surgical procedures is promising but untested.
Further study of the dynamics of hemorrhage and additional results are
needed prior to making a decision on how to divide patient management
into the two categories of surgical and nonsurgical treatment.
|