|
Irreversible disability is traditionally associated with
cerebral vascular disease (CVA) and it is under-estimated that there are
2 million fatalities from vascular disease every year. Although medical
measures help in acute ischemia, they are only palliative in an
established stroke and in prevention of a repeat stroke.
Cerebral revascularization is the goal. Of late, there is
an increasing acceptance of surgical revascularization even in
acute episodes.
Etiology:
Arterial thrombosis and atherosclerosis with
occlusion of the cerebral arteries is the single most common cause of
stroke in more than 50 percent of patients.
Thirty to 50 percent of the cases have had previous
transient ischemic attacks.
Other rarer
causes include trauma to the
carotid/vertebral arteries, collagen diseases, moyamoya disease,
fibromuscular dysplasia, vasospastic conditions (SAH, migraine etc), and
the conditions which alter the rheological properties if the blood such
as, polycythemia, leukemia etc.
There are risk factors, such as, hypertension,
hypercholesterolemia, atherselerosis, cardiac abnormalities, diabetes
mellitus, obesity, and lack of physical activity..
Clinical features:
The earliest sign of the CVA is the transient ischemic
attack (TIA). The other findings may be a history of diplopia,
atherosclerosis of the retinal arteries, speech difficulties, motor or
sensory changes, abnormalities of cerebellar function etc.
Transient ischemic attacks (TIAs)
are usually described as related to the carotid or vertebral-basilar
arterial systems. It is defined as an acute cerebral dysfunction
resulting from vascular problem, which recovers within 24 hours.
Carotid territory:
The classic history for transient ischemic attack in the carotid
system is one of swift onset of contralateral weakness or numbness of the
arm or leg. Dysphasia occurs if the dominant hemisphere is involved.
Impaired vision of the eye on the side of the diminished carotid flow
takes place. This clinical phenomenon usually indicates a decrease in
regional cerebral perfusion, and produces a neurological deficit, the
onset of which is usually sudden with gradual progression of the
symptomatology.
Carotid artery occlusion is caused by atherosclerosis,
arteriosclerosis and atheroma, and is compounded by the extension of
cholesterol and calcium deposits into the branches of the common carotid
artery, specifically the internal carotid artery.
There are five items, which would bring one to suspect
involvement of the carotid arteries:
1. Blindness in one eye during the TIA attack.
2. Emboli in the retinal vessels.
3. Bruit over the carotid artery.
4. Significant lowering of the retinal arterial pressure on
the affected side.
5. Any sign of retinal artery ischemia.
Vertebral-basilar territory:
Damage to this system is also characterized by a very swift
onset of symptoms with neurological phenomenon such as ataxia,
monoparesis, hemiparesis, quadriplegia, numbness (frequently shifting
from one side to the other), vertigo, defects in either visual field,
diplopia, dysarthria, aphasia and occasionally, clouding of
consciousness. Vertigo is perhaps the most common symptom of TIA in this
distribution.
Prolonged reversible ischemic defect
(PRIND) is one where the signs and symptoms lasts for a week followed by
recovery.
Progressing stroke (PS) is one where
the deficit continues to progress in a stepwise fashion despite adequate medical
therapy.
Stroke is a sudden, unheralded
progressive neurological deficit due to a vascular pathology; the deficit
becomes complete in few hours. It is usually associated with altered
sensorium and hypertension.
Associated carotidynia (pain over the carotids)
suggest a carotid pathology.
Investigations:
TIAs
should be regarded as an emergency. The risk of stroke is greatest in the
weeks following TIA and patients should be referred for further
investigations at the earliest. The initial evaluation of a
patient in whom a transient ischemic attack is suspected should include
laboratory tests, electrocardiography, and imaging studies. Since imaging
of the head may reveal a nonischemic cause, such as a tumor or subdural
hematoma, and may provide information about the cause of ischemia, it is
recommended that CT or MRI of the head be part of the evaluation of all
patients. Doppler ultrasonography or other noninvasive investigations of
the carotids should be performed rapidly, ideally within 24 hours.
|
CT will demarcate the area of ischemia and
exclude hemorrhage and also the previous infarcts. Small lacunar
infarcts suggest an arteriolar pathology (of the penetrating branches
of major cerebral arteries). Wedge shaped infarcts suggest
thromboembolism. Ill defined border zone infarcts suggest hemodynamic
ischemia. Good recognition of a perimesencephalic pattern of
hemorrhage is possible on unenhanced CT, and CT
angiography accurately excludes
and detects aneurysms and AVMs. DSA can be
withheld in patients with a perimesencephalic pattern of
hemorrhage and negative CT
angiography. CT angiography of cervical vessels
reveals enough vascular detail to be useful as a diagnostic
screening method in patients with presumed atherosclerosis
of the carotid bifurcation.
|
|

|
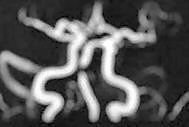
|
|
CT angio-ICA occlusion
|
MRA- Basilar occlusion
|
|
MRI is more
sensitive, in particular for previous hemorrhage. MRAngiography, a
noninvasive test, permits the visualization of blood flow in vessels
without the need for catheters or contrast agents. The technology
can yield information regarding collateral blood flow and is nearly as
effective as conventional angiography in estimating disease at the
carotid bifurcation. It is suitable for replacing the invasive
conventional angiography method in most cases.
Further technical developments with regard to spatial
resolution are still required for improved visualization of
small vessels and terminal branches of intracranial vessels.
Ultrasonography, being cost
effective, should be used as a screening tool to exclude patients with no
carotid artery disease from further testing.
B-mode
imaging
provides images of various levels, or planes, enabling the creation of a
three-dimensional image of the carotid artery wall and surround
structures. This technique provides information on the type and
extent of arterial damage, but blood clots sometimes do not appear and
the method cannot distinguish a severely narrowed from a completely occluded
artery.
|
Doppler testing
measures the speed of blood flow through an artery. Two types of
Doppler ultrasound are used to obtain information on the velocity of
blood flow in the carotids. In pulsed Doppler, the probe is placed over
one spot on the neck over the carotid, and timed measurements are taken
to determine the speed of blood flow in the artery. In continuous wave
Doppler, a probe is moved along the neck over the course of the
carotid, and the velocity of blood passing along the vessel beneath the
probe is averaged out.Duplex ultrasound (DUS) combines B-mode
imaging and pulsed Doppler ultrasound to provide more detail on the
condition of arteries than either test alone can provide. When
performed in settings in which the results have been consistently well
validated by comparison with angiography, it is an accepted and
accurate technique, but there is risk of calling a high-grade stenosis
total occlusion (1% to 14% false-positive rate).
|
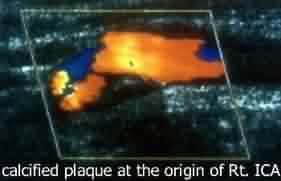
|
However,
its reliability is highly dependent on the technician. The recent
availability of ultrasound contrast agents helps to distinguish between
pseudo- and true occlusions, improves ultrasound images and should help
to reduce operator variability. Color and spectral Doppler ultrasound are
now recognized as the best screening tests for carotid artery stenosis.
The evidence for its use as the sole diagnostic imaging modality prior to
carotid endarterectomy is examined. Supplementary imaging is especially
advisable when results of DUS are technically limited.
Transcranial
Doppler (TCD)
assesses intracranial arterial flow in the distal ICA, the middle,
anterior, and posterior cerebral artery stems, and ophthalmic artery.
Hemodynamic significance extracranial and intracranial ICA occlusion and
the availability of collateral circulation may be studied
satisfactorily.Transorbital (ophthalmic artery), submandibular (distal
ICA), transtemporal (Anterior cerebral. Middle cerebral and posterior
cerebral), and foramen magnum (posterior circulation) approaches are
employed for a comprehensive assessment. Serial TCD examination may
reveal dynamic changes in cerebral circulation that may be missed on a
single MRA study. Preoperative TCD can be used to identify patients who
do not require a shunt during carotid endarterectomy. In acute ischemic
stroke, TCD can be used to elucidate stroke mechanisms, plan and monitor
treatment, and determine prognosis. In an era when stroke is increasingly
being recognized as an emergency requiring immediate treatment, TCD may
be capable of providing rapid information about the hemodynamic status of
the cerebral circulation, within the time frame of the rather small 'therapeutic
window'.
Ophthalmodynametry
and oculoplethysmography offer an indirect indication of
ipsilateral carotid occlusion. Ischemia of the macular region is
necessary to produce transient monocular blindness and local retinal
blood flow has been reduced to the flow threshold of
electrical failure in patient. Ophthalmic artery flow reversal is not
only quite specific for severe ICA disease, and also provides additional
hemodynamic insights (i.e., the inadequacy of other collateral channels
such as the anterior communicating artery.
|
Conventional four vessel arteriography
should include cerebral, carotid and arch studies and with cross
carotid compression. One may also find post-stenotic lesions of the
bifurcation, patency of the anterior cerebral vessel, absence of the
vertebral artery, occlusion of the vertebral artery and partial
occlusion of the internal carotid vessels. Internal carotid patency
along with cross filling of the anterior, middle cerebral and the posterior
communicating vessels may be evaluated. However, conventional
arteriography fails to demonstrate some vascular mural
changes that may intervene in the development of clinical
manifestations, such as intraplaque hemorrhage and thrombus
attached to the arterial wall. These mural changes may be identified
with duplex ultrasound and CT arteriography. With increasing
experience with noninvasive imaging, angiography may be required less
often. Clinicians should be cautious when using contrast enhanced MRA
alone for surgical decision making in CEA candidates because a
significant number of patients may be misclassified. The rate of
misclassification is reduced when the results of contrast enhanced MRA
and duplex Doppler ultrasound are concordant. Further study is needed
to evaluate the benefits and risks of endarterectomy without
angiography.
|
|
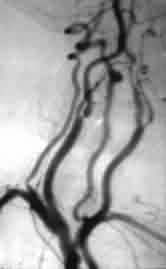
|
|
4 vessel angiography-ICA
occlusion
|
|
Doppler
CO2 / Acetazolamide (diamox) test: CBF measured early after acetazolamide
administration could be useful to confirm the clinical
diagnosis of TIA. No increase in CBF during hypercapnia or
following acetazolamide suggests that the cerebral arterioles are
maximally dilated and the procedures to improve the blood flow, such as
EC-IC bypass will not help.
|
Single-photon
emission computed tomography (SPECT) studies combine nuclear
medicine with computed tomography. Used in early hours after
infarction, cerebral SPECT is able to reveal a deficit in local blood
flow before changes appear on CT or MRI. However, SPECT does not
reliably distinguish between hemorrhage and infarction, and it is
unclear whether the method will predict the potential for clinical
recovery. In patients who are marginal candidates for
endarterectomy, the hemodynamic effect of stenosis on cerebral
perfusion may be assessed with SPECT, and is useful in
predicting neurological outcome in ischemic stroke patients.
Positron
emission tomography (PET) can be used to measure cerebral
blood flow (CBF), cerebral blood volume (CBV), and metabolism (CMR).
Patients with low CBF and high oxygen extraction (OEF) have compromised
cerebral circulation and are expected to benefit from
revascularization. These studies are helpful in an established
stroke, and to differentiate between flow related TIAs, and
thromboembolic TIAs. None of these applications is
sufficiently widely used in the clinical practice of neurology to
provide a recommendation.
|
|
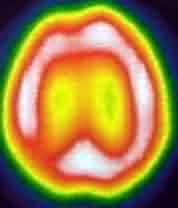
|
|
SPECT-Rt.parietal ischemia
|
|
Surgical revascularization:
a) Carotid territory:
The overall risk of a stroke, following a TIA, is about 12%
during the first year, rising to 30% within 5 years.
The risk is higher, about 30%, with internal carotid
stenosis, whereas those with complete carotid stenosis are unlikely to
develop a stoke in the same territory.
The stenosis will progress in more than 50% of cases over
the subsequent 1-5 years.
The role of carotid endarterectomy is now well
established.
Various other procedures are being tried with no
satisfactory evidence based benefit.
1) Carotid endarterectomy:
Indications:
The ideal patient for carotid endarterectomy is one who
presents with a history of transient ischemic attack, hemispheric or
retinal and has no neurological deficit on physical examination and
who has a stenotic lesion at the orifice of the internal carotid
artery.
TIAs of embolic origin do well with endarterectomy, whereas,
the flow related ones do not do as well.
Patients with stroke within previous 6 months with 70%-99%
stenosis of ipsilateral ICA.
Patients with lesser stenosis but an ulcerative plaque.
Traumatic occlusion of the carotid and spontaneous
dissection of the carotid may be considered for endarterectomy.
Acute stroke, when the procedure can be performed within 2-3
hours of the onset (with no evidence of infarct on CT), is a
controversial indication, becoming less and less controversial of late.
Progressive stroke despite effective medical measures, and
occlusion of an asymptomatic carotid, while investigating the symptomatic
carotid or vertebro basilar territory are other controversial
indications.
Surgical
technique:
Preoperative counseling is
important as preparation of the patient mentally helps a great deal in
overcoming the fear and anxiety. If the patient is on antiplatelets
already, ASA may be continued through the procedure. But Clopidogrel is
stopped two days pre operatively in our practice.
In
the operative room patient is sedated with 1mg Medzolam intravenously
after securing venous access and arterial pressure monitoring catheter.
Arterial pressure is monitored continuously as fluctuation during the
procedure can affect the cerebral circulation.
Positioning
of the patient is extremely important as hyperextension of the neck may
kink the vetebrals which will be the likely source of blood supply during
cross clamping of ICA.
Intraop
monitoring: 1) EEG: Electroencephalography is a sensitive
detector of cerebral ischemia and a valuable tool for determination of
need for shunting during carotid endarterectomy. A statistically
significant increase in intraoperative stroke rate is associated with the
development of an abnormal EEG (1.1%), contralateral internal carotid
artery occlusion (1.8%), and the combination of both abnormal EEG and contralateral
internal carotid occlusion (3.3%).
2)
SSEP:
If available, use of SSEP is the ideal monitoring under general
anesthesia. Registration of SEPs is simple to perform and indicates with
a high sensitivity and specificity critical cerebral hypoperfusion during
cross-clamping. Progressive reduction of up to 50% of N20, P25 amplitude
is suggestive of ischemia. SSEPs not only help to identify patients with
insufficient collateral blood flow who benefit from specific cerebral
protection, such as shunt, but also to avoid improper and hazardous
application of these measures in patients with sufficient cerebral
perfusion. In addition, correct shunt function is immediately indicated
by recovering potentials.
3)
Stump pressure measurement: Measurement of ICA stump backpressure
helps in deciding on the need for a shunt. Reports suggest that
surgery without a shunt when the ICA back pressure is low(less than 50
mm/hg), produce significant deficit.
4)
Transcranial Doppler (TCD): The perioperative stroke rate can be
reduced by appropriate measures, taken by the surgeons, based on findings
of TCD monitoring.TCD will help detect high blood flow velocities.The
clinical significance of bilateral flow velocity increases soon after
surgery is uncertain, but very high blood flow velocities might be a
signal for cerebrovascular hyperperfusion. In those patients, increased
postoperative surveillance is recommended.
None
of the haemodynamic criteria by stump pressure and TCD are absolutely
reliable in predicting the need for carotid shunt. The usefulness of
monitoring cerebral function during the procedure is closely related to
the experience of the surgical team. No one method of monitoring in
selective shunting has been shown to produce better outcomes. No
prospective randomised or quasi-randomised trials have been performed and
the conclusions therefore remain unchanged. Recommendations whether to
practise cerebral monitoring or not, and what method should be used for
this purpose, cannot be given presently.
Procedure:
We
prefer regional anesthesia. It obliviates the need for extensive
introperative monitoring for cerebral ischemia. Careful assessment of
cerebral perfusion can be made by direct interaction with the patient.
This is by far the best mode of assessment of cerebral perfusion when
compared to other modalities like TCD and SSEP. Regional anesthesia is
obtained by both superficial and deep cervical block. Deep cervical block
involves infiltration of local anesthetic agents around C2, 3, and 4 at
the exit foramina. Superficial block involves infiltration around the
cervical plexus at the lateral border of the sternocleidomastoid muscle
at the level of external jugular vein. We use approximately 40cc of
0.375% Bupivacaine for this as the anesthetic effect lasts as long as
6-8hrs.
Just
before making the incision patient is given Fentanyl 25mg intavenously
for additional sedation. Incision is made parallel to anterior border of
sternomastoid from the level of thyroid in cartilage to just below
mastoid process.
|
A long
high incision is made along the anterior border of the sternomastoid
muscle, almost to the mastoid tip. It often necessitates the division
of a branch of the great auricular nerve as it crosses the anterior
margin of the sternomastoid muscle, resulting in usually temporary ear
and/or lower jaw skin numbness.
The
plane beneath the investing fascia of the neck is followed under the
sternomastoid muscle, and after the sternomastoid muscle is mobilized,
blunt self-retaining retractors are used to expose the underlying
areolar tissue, beneath which lies the internal jugular vein and
carotid sheath.
Sharp
dissection is continued, first skeletonizing the internal jugular vein
and the common facial vein. The common facial vein and any nearby vein
emptying directly into the internal jugular vein are divided so that
the jugular vein can be retracted posteriorly, exposing the carotid
arteries beneath.
The
carotid sheath is opened to expose the common carotid artery above the
upper margin of the overlying omohyoid muscle, and dissection proceeds
distally to the bifurcation and to the external and internal carotid
arteries.
It is
necessary at all times but particularly at this stage to handle the
tissues gently and disturb the arteries from their bed as little as
possible, especially when manipulating the internal carotid artery near
the plaque. It is not wise to palpate plaque within the internal
carotid artery, which is usually located at the site where the artery
is most adherent to adjacent tissue, because of the risk of dislodging
an intraluminal thrombus into the cerebral circulation. Sinus
nerve at the bifurcation of the carotids is blocked with 1% lignocaine
during carotid dissection.
During
distal exposure of the internal carotid artery, care is taken not to
injure or excessively manipulate hypoglossal, vagus, and accessory
nerves, although the latter is high and posterior in the carotid sheath
and infrequently exposed. The hypoglossal nerve, which is routinely
exposed, descends deep to and beneath the digastric muscle and curves
forward superficially to the external carotid artery; it often can
readily be traced to this location by following a branch, the
descendens hypoglossi, proximally from its course within the carotid
sheath.
Vessel
loops are then passed around the common, external, and internal carotid
and superior thyroidal arteries, and heparin (75 I.U/kg) is
administered. Mean arterial pressure has to be maintained around
100mmHg before carotids are clamped. After three minutes, carotids will
be cross clamped sequentially. Care should be taken to palpate the
artery at the precise point where one intends to put the clamp and one
should be certain that it is below a hard, calcified plaque, which
could easily fracture. Internal carotid first followed by common
carotid and external carotid. Superior thyroid artery which arises from
external carotid close to bifurcation has to be occluded
separately.
At
this stage careful neuro monitoring is done by anesthetist by assessing
the level of consciousness and motor activity. Any deterioration in the
assessment at any stage will be an indication for shunt insertion to
protect the cerebral circulation.
An
arteriotomy is extended proximally from the common carotid artery, with
care being taken to keep it in the middle of the lateral exposure of
the internal carotid artery and away from the apex of the carotid
bifurcation. Internal carotid clamp is opened to asses the back
bleeding which is another indicator of cross circulation.
The
atheroma is separated, particularly at the distal end of the internal
carotid endarterectomy, followed by complete removal of all small,
loosely adherent circumferential plaque remnants from the
endarterectomy site. Constant heparinized saline irrigation is recommended.
The
greatest of care should be taken with the upper end of the
endarterectomy, and if the plaque has not come out smoothly, the
surgeon should be prepared and able to open the internal carotid artery
another 5 mm in order to improve the distal repair. Any significant
distal intimal step-off or shelf not firmly adherent to the arterial
wall should be tacked down with 7-0 monofilament sutures. A partial or
circumferential plaque should never be pulled down and away from above
the level of the arteriotomy within the internal carotid, because a
loose distal intimal attachment, vulnerable to subintimal dissection
and carotid occlusion, can neither be fully appreciated or properly
repaired in this location. The atheroma extending up the external
carotid artery is mobilized circumferentially, and the plaque is
everted from the arterial lumen. Microsurgical method increases
the precision and safety of every aspect of carotid endarterectomy,
including complete plaque removal, prevention of intimal flaps, nonstenosing
arteriotomy closure.
After
irrigation of the area the arteriotomy is closed with 6.0 prolene.
Before the final arteriotomy suture, the internal carotid artery
temporary clip and the common carotid artery clamp are removed
momentarily in turn, allowing air to be expelled from the nearly
repaired arteriotomy. If the internal carotid artery is small
which is the case in small built females a Gortex patch can be used as
angioplasty.
Patients
with complicated recurrent atherosclerosis can be treated with
endarterectomy and patch grafting, but interposition vein grafts should
be considered in cases in which the vessels are extensively damaged by
the
|
|
|
|
Skin incision
|
|

|
|
Exposure of carotids
|
|

|
|
Arteriotomy
|
|

|
|
Removal of atheromatous
plaque
|
|
|
|
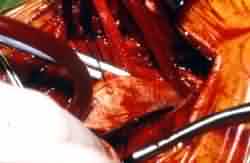
Endarterectomy completed-javed
shunt being inserted
|
|

|
|
Arteriotomy closure with a
patch
|
|

|
|
Completed closure with
a patch
|
|
|
|
Atheroma
|
|
recurrent plaque or with an unexplained
thrombus at the site of previous endarterectomy. Clamps are sequentially
released after arteriotomy closure. External carotid circulation is
established first as any small debris from the operative site may be
released into extra cerebral circulation. Internal carotid clamp is
released at the end. If the haemostasis is satisfactory reversal of the
heparin may not be required.
The
wound is closed after placing a suction drain as the wound is likely to
ooze due to preoperative antiplatelet therapy.
Post
operatively, close neurological observation is necessary by a dedicated
nurse. Post operatively patient can be started on oral fluids after two
hours and all the medication as before including anti platelets. There is
no indication for routine anticoagulant therapy.
Use
of Shunt:
The only method currently accepted by all surgeons to achieve cerebral
protection, is the use of shunt during carotid endarterectomy. The
relative risk of shunting versus not shunting during carotid
endarterectomy was analyzed by Sundt TM jr et al retrospectively in 1935
cases undergoing carotid endarterectomy for carotid ulcerative stenosis.
The need for shunting was based on a correlation between
electroencephalographic changes and a fall in cerebral blood flow below
the critical level required for adequate perfusion during the period of
carotid occlusion. Patients were divided into four risk categories for
surgery, based on medical and neurological risks and angiographic
findings. Shunts were required in 30% of the low risk group and 56% of
the high risk group. Based on the severity of reductions of cerebral
blood flow during the period of carotid occlusion it is concluded that
12% of all patients would have sustained a major deficit, 15% a minor or
transient deficit, and 20% a transient deficit without shunting. The risk
of shunting 792 cases in this series was 0.5%. Overall minor morbidity,
major morbidity, and mortality each approximated 1% in this series.
It has
been suggested that external shunts, placed between the common carotid
artery and the internal carotid artery (ICA), is safe and efficacious in
cases that do not permit the placement of an internal shunt. A new
type of temporary extraluminal shunt, connecting the femoral to the
internal carotid artery with the interposition of a roller pumps to
regulate the blood flow has been reported. This method allows one to
perform carotid endarterectomy without interrupting the blood flow to the
brain.
Complications:
The operative mortality and morbidity is 1-5% in
various studies.
The majority of strokes after carotid endarterectomy
are thromboembolic and many can be traced to technical failures, such as
the creation of an intimal flap, incomplete plaque removal, or the
creation of kinking or stenosis during arterial closure. Severe and
damaging cross-clamp ischemia in patients with poor collateral flow to
the ipsilateral hemisphere underlies fewer postoperative strokes but may
be detectable with intraoperative cerebral monitoring.
Intraoperative shunts may reduce the risk of
stroke in this subgroup of patients; some surgeons use intraoperative
shunts in all patients.
Microsurgical method increases the precision and safety of
every aspect of carotid endarterectomy, including complete plaque
removal, prevention of intimal flaps, nonstenosing arteriotomy closure,
and intraoperative shunt insertion when necessary.
Restenosis occurs in about 20%; use of
dacron or vein patch during arteriotomy closure when ICA internal
diameter is <5mm is recommended.
To improve the prospects for postoperative carotid artery
patency, use of antiplatelet therapy both preoperatively and
postoperatively is advised.
|
Results:
The first report from the North American Symptomatic
Carotid Endarterectomy Trial (NASCET), which concluded
that carotid endarterectomy is highly beneficial to patients with
recent hemispheric and retinal transient ischemic attacks or
nondisabling strokes and ipsi-lateral high-grade stenosis (70-99%) of
the internal carotid artery, reported a cumulative 9% risk of any
ipsilateral stroke over 2 years in the surgical group of 328 patients.
In the perioperative period, 18 surgical patients (5.5%) had
cerebrovascular events: 12 minor (3.7%), 5 major (1.5%), and 1 fatal
(0.3%).
|
|
|
|
|
pre-op
|
post-op
|
|
|
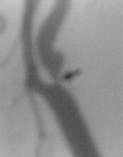
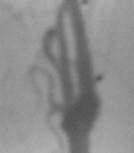
angio - ICA stenosis
|
|
|
In that study, three of the major strokes were due to
carotid occlusion in the early hours after endarterectomy, and 10 of the
minor strokes were also delayed in onset and were presumably embolic.
MRC European Carotid Surgery Trial (ECST) suggests
the patients with a stenosis <30% need not be operated; benefit
of surgery in those with 30-69% stenosis is not conclusive.
2)
Extracranial to intracranial (EC-IC) bypass:
Superficial
temporal to middle cerebral artery bypass (STA-MCA): Small vessel
disease (about 20% of all ischemic patients), middle cerebral artery
occlusion where endovascular thrombectomy has failed or is not feasible,
and total ICA occlusion may benefit from a EC-IC bypass procedure.
This
procedure was popular in the 70s. It involves anastomosis of the
superficial temporal artery to one of the cortical branches of the middle
cerebral artery.
The
skin in incised over the proximal STA, just above the zygoma. The STA has
at least two major branches (frontal and parietal) and they should both
be followed distally. The STA is dissected with a small cuff of tissue to
prevent vessel injury. The larger branch is freed.
A small
craniotomy centered over the sylvian fissure is made and a recipient
artery is selected and dissected from the archnoid to follow anastomosis.
The STA
is ligated and divided and the proximal STA is occluded with a temporary
clip.
The
recipient art is transiently occluded between two temporary clips and an
end to side anastomosis is performed with 10-0 monofilament suture.
Cerebro-protective techniques, including hypothermia and barbiturates
help.
This
provides initial flows of 25-50ml / minute; with time the bypass may
mature, allowing enlargement of STA and delivery of a higher
flow. Occipital artery to the intracranial circulation has also been
tried.
Interposition
vein graft is
recommended by some, especially when STA is not satisfactory.
Complications
include aneurysmal dilatation and rupture of the graft and emboli from
the graft site.
Anecdotal
reports and uncontrolled patient series suggested that STA-MCA bypass may
be beneficial. However the National institutes of health (NIH) study in
1985 concluded that these procedures do not help in preventing a stroke,
despite an overall graft patency rate of 96% and low surgical morbidity.
They may have a place when everything else fails in the highly selected
patients where the metabolic reserve studies suggest a compromised
CBF. In the treatment of inoperable ICA giant aneurysms where the
risk of ischemic complications due to ICA ligation is high, EC-IC bypass
may be used as a prelude to ICA ligation. Chronic biochemical
abnormalities due to brain ischemia may improve after cerebral
revascularization.
Vertebro-basilar
territory:
The
cerebellar infarctions carry poor prognosis with an acute mortality rate
of 20-30%. They are mostly due to poor flow (due to stenosis and poor
collaterals) due to diffuse atherosclerosis of the vessels. Medical
therapy is the first line of therapy. Several procedures have been tried
in those with persisting symptoms. There has been no randomized
study.
The
simplest procedure, perhaps, is carotid endarterectomy if a significant
stenosis is found while investigating a vertebrobasilar TIA; the stenosed
carotid may be asymptomatic. It is most readily accepted if the angiogram
shows filling of the posterior cerebral artery via the stenotic ICA, or
filling of the posterior circulation from the ICA because of vertebral
occlusion or a persistent hypoglossal or trigeminal artery.
Good outcome
with vertebral endarterectomy, which is similar to carotid
endarterectomy, has been reported. Posterior circulation bypass (
occipital artery to PICA for occlusion proximal to the PICA and a
superior cerebellar artery or P1 segment of the posterior cerebral artery
anastomosis for lesions at the mid or distal basilar) have been
described.
Extra
cranially, the left subclavian artery, next to the carotids and the
vertebrals, is most commonly involved in atheroscleorosis. Subclavian
syndrome is most commonly treated by carotid-subclavian bypass. The
cervical vertebral artery may occasionally compressed by cervical
osteophytes. Anterior cervical decompression ,reportedly, helps.
b)
Indirect revascularization procedures:
|
Intracranial nonatherosclerotic
occlusive diseases form a heterogeneous group with diverse
pathogenesis. Moyamoya is the commonest. Moyamoya is a progressive
occlusive cerebrovascular disorder characterized by bilateral stenosis
and occlusions of intracranial arteries with extensive
neovascularisation at the base of brain. It was first described in
Japan and now reported from all over the world. Genetic linkage studies
and study of the factors possibly involved in its pathogenesis have
shed new light on this disease. There is some suggestion that the
pathogenesis may vary between races. In pediatric-onset moyamoya
disease, asymmetrical involvement of bilateral ICAs and PCAs was
common, and the ipsilateral ICA and PCA tended to be predominantly
involved.
|
|
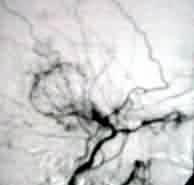
|
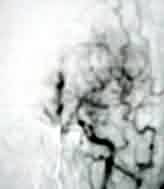
|
|
Moyamoya-angio(lat)
|
Moyamoya-angio(AP)
|
|
Patients
present with stroke or intracranial hemorrhage due to bleeding from the
friable vessels with major morbidity and mortality. Children usually
present with cerebral ischemia. Intracranial hemorrhage is common in
adults. Without treatment, there is progressive deterioration of
neurologic function and re-hemorrhage.
Treatment
of all these patients with nonatherosclerotic occlusive diseases is
similar to that of moyamoya patients. Medical therapy with vasodilators,
steroids, and antibiotics is only minimally effective. Various surgical
procedures have been tried with mixed results.
STA-MCA bypass in
adults, encephloduroarteriosynangiosis, encephalomyosynangiosis, and
encephaloomental-synangiosis in children are the available procedures.
Direct superficial temporal artery to middle cerebral artery bypass is
considered the treatment of choice, although its efficacy, particularly
for hemorrhagic disease, remains uncertain.
Encephalomyosynangiosis
involves
direct placement of temporalis muscle on the cerebral cortex. This may
induce seizures.
Encephloduroarteriosynangiosis developed by Matsushima
is popular. STA is mobilized and placed directly on the cortex.
The free artery with a cuff of surrounding soft tissue is simply sutured
to the dura.
Encephaloomental-synangiosis
(omental-cerebral transposition) may provide an alternative. The omentum
is mobilized from the abdomen in a subcutaneous tunnel and placed over
cerebral cortex as a pedicle graft or, as a free graft with microsurgical
anastomosis of the gastroepiploic artery and vein to the STA and the
superficial temporal vein. Omentum contains biologically active
substances taking part in neural transmission and has angiogenic and
neurotrophic action.
Combined
procedures give the best results. Spontaneously developed leptomeningeal
anastomosis might be the key factor for the efficacy of indirect bypass
in elderly patients with moyamoya /stenotic cerebrovascular disease. There
is no controlled study available. Satisfactory results are reported.
Surgery for Acute Stroke:
To
appreciate the role of surgery in acute stroke, it is imperative to
understand the pathophysiology
of stroke.
Recently,
the effectiveness of many medical therapies for brain edema
and the subsequent increased ICP has been challenged. Recent
studies have shown a very high mortality rate despite aggressive medical
treatment strategies to lower ICP, which have included osmotherapy,
hyperventilation, barbiturate administration, hypothermia, and
anticoagulation therapy guided by ICP monitoring. Compared with
these, decompressive craniectomy seems to result in a much better
outcome; surgical decompression should be performed before inducing deep
barbiturate coma in these patients; a surgical decompression performed
after failure of ultrahigh barbiturates is too late.
1)
Craniectomy and decompression:
A
decompressive procedure in selected stroke patients is the most practiced
surgical intervention in acute strokes. Any surgery should be effective,
rational, and safe. An ipsilateral decompressive surgery fulfils all three
criteria. The procedure is, certainly, simple and safe.
The
intracranial mass effect can be compensated without an increase in ICP by
resorption of cerebrospinal fluid (CSF) and by shifting CSF into the
spinal canal. When the reserve spaces become completely exhausted, mass
effect leads to an exponential increase in ICP. The equation is expressed
by the pressure-volume curve. The studies provide evidence that
decompression leads to a shift to the right of the pressure-volume curve
and, therefore, to a massive increase in compliance and a reduction of
ICP. The rationale for decompressive surgery is supported by these
studies.
The
available results suggest the effectiveness of decompressive surgery as a
salvage procedure.
Associated
illnesses and the attitude of the family members to accept a severe
neurological deficit, especially in developing countries where there is
no adequate rehabilitation centers, must also be considered. As in any
surgery, patient selection, and meticulous postoperative management play
a major role in the outcome. Associated illnesses must be attended to.
|
Cerebral infarcts: A subgroup of patients with a large cerebral infarct
qualifies for a decompressive procedure to prevent uncal herniation and
death. This generally follows a massive multilobar infarction. They
develop space-occupying cerebral edema with subsequent herniation
and death. It is well recognized that cerebral edema after
large MCA infarcts occurs in up to 10% of all patients. Even
under full supportive therapy, the mortality rate for this
distinct syndrome of malignant MCA infarction is roughly
80%. If there is no satisfactory recovery with aggressive medical
therapy within few hours, surgical hemicraniectomy and decompression
should be considered in patients with malignant cerebral edema.
|
|
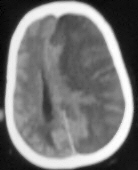
|
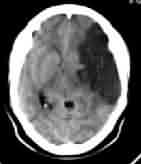
|
|
Multiteritory infarct-CT
|
Malignant MCA infarct-CT
|
|
The aim
is patient salvage during the acute period of brain swelling. None of the
available medical therapies provide substantial relief from the oedema
and raised ICP, or at best, they are temporizing in most cases.
The
technique
is simple. Judicious timing is the key for success. Craniectomy should be
performed early, before severe impairment of brain perfusion occurs.
Computerized tomography might be able to predict the dynamics of the
ensuing clinical course to assist in indicating early intervention in
some patients. There are no systematic reports about quantitative
analysis of the size of craniectomy required to be effective.
Traditionaly, craniectomy is planned according to the area of infarct. A
wide craniectomy with a duraplasty is, routinely, recommended.
Ideally,
as described by Cushing,, the craniectomy should extend to the
base and include drilling of the sphenoid ridge for adequate
decompression. Achieving a decompression down to the floor of the middle
fossa (subtemporal decompression) seems to be important in this surgical
technique, because this procedure relieves pressure from the basal
temporal lobe. Good results with this technique were reported even though
this form faces the risk of temporal lobe herniation and necrosis. As the
dura is opened, pale infracted brain herniates out. The herniation may
subside with hyperventilation and osmotherapy. The author recommends
excision of persistent herniated brain to prevent strangulation and
necrosis. Duraplasty is performed with either silastic lyodura or
pericranial grafts.The graft is secured with sutures in a way that allowed
the initial incision to spread not more than 2 to 3 cm.This achieves
smooth bulging rather than fungus like herniation of brain into the
craniectomy, avoiding shearing injuries, impairment of venous drainage,
and enhancement of cerebral edema. It is also recommended that in
bifrontal craniectomy, the sphenoidal ridges and the anterior walls of
the middle cranial fossa be preserved to prevent temporal lobe forward
migration. Some groups have suggested resection of infarcted and
even noninfarcted brain tissue. Some others recommend resection of the
infarcted cerebral tissue and a temporal lobectomy. Japanese surgeons
recommend additional excision of the hippocampal gyrus also to relieve
peduncle compression, and blockage of cerebrospinal fluid circulation.
Another small group of surgeons include a slit in the tentorium to
relieve further compression. The author recommends a subdural positive
pressure drainage at the end of the procedure helps to facilitate CSF
drainage if required post operatively and may be incorporated with an ICP
monitor.
The
author recommends that conservative measures, such as hyperventilation
and osmotherapy, must be tried before surgery is considered and that the
decompressive procedure is performed prior to frank clinical deterioration.
Ideally, the patient should be young with a GCS of >5, and no serious
systemic illness, and must have a supporting family. There may be
occasions when the patient selection needs to be individualized. The
procedure is mainly to give the maximum chance to preserve life. There is
a group of reluctant surgeons who feel, a decompressive procedure do not
alter the final outcome. Discouraging outcomes in patients do not
invalidate the method; good results confirm its usefulness. The increase
in brain edema after decompressive craniectomy led to a discussion in the
neurosurgical literature and to questioning the usefulness of the
procedure when treating severely brain-injured patients. Brain edema only
increases if the brain is already irreversibly severely damaged. Such
patients have a poor prognosis, which is no argument against
decompressive craniectomy. At present decompressive surgery might be the
most promising therapeutic option. For decisive answers, randomized,
controlled clinical trials are needed.
Cerebellar
infarcts:
Older hypertensive men with diffuse atherosclerosis are commonly
affected. Previous myocardial infarction or cerebral infarcts are often
noted together.Cardiogenic embolus is thought to be the etiological
factor in about 50% of the cases. Trauma is an occasional cause.
Pediatric group is being recognized increasingly.
The
infarct is usually unilateral, and the PICA territory (posteroinferior
aspect of the cerebellum) is the site involved. The superior cerebellar
artery is an uncommon site of occlusion. Extensive infarction may involve
1/3 or1/2 of the hemisphere and brainstem compression. 25% of them are
hemorrhagic.
|
A history of posterior
circulation TIA may alert the physician. In massive infarction, the
patient becomes progressively obtunded. When the aggressive medical
therapy fails in a reasonable time, surgical decompression should be
considered.Today the only indication that seems to be widely accepted
for performing decompressive surgery is in cerebellar infarction with
continuous clinical deterioration, as shown in several large trials.
Our
practice is to perform a simple suboccipital craniectomy in prone
position with resection of infarcted tissue. The posterior arch of the
atlas is removed for wider decompression. Ventricular drainage is
established for concomitant hydrocephalus and converted to a shunt if
necessary at a later stage. It must be understood that patients with
brainstem infarction have poor outcome; but, brainstem compression is
potentially reversible.
|
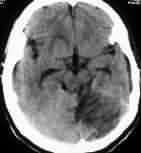
|
|
|
Cerebellar infarct-CT
|
|
2)
Revascularization procedures:
The role of
revascularization procedures in acute stroke is still in the experimental
stage. Emergency carotid endarterectomy is a controversial indication,
becoming less and less controversial of late. Patients must have
angiographically demonstrated lesion and no infarction in a CT. There is
no randomized trial. Tissue plasminogen and interventional endovascular
procedures are more often preferred.
Recent
reports suggest moderate success of emergency carotid endarterectomy in
patients a) with cresendo TIAs, b) with severe stenosis in angiography,
and c) with disappearance of a previously auscultated bruit, presumably
indicating acute occlusion. The presence of good collateral flow is a
favorable prognostic sign. The technique is the same as in elective
endarterectomy. Clinical results are best in patients with mild to
moderate deficit and a rapid course from onset of deficit to surgery.
Despite
excellent postoperative results, the outcomes in patients after
STA-MCA anastomoses are not better than the results from
medically treated patients. Other procedures such as posterior
circulation bypass, vertebral endarterctomy, arterial anastomses, and
corrction of subclavian steal have not been tested sufficiently.
Hemorrhagic
transformation
is frequently seen on CT scans obtained in the subacute phase of ischemic
stroke. Its prognostic value is controversial. Hemorrhagic transformation
of an ischemic infarct is managed the same way as an ischemic infarct is.
Hemorrhagic
stroke: (discussed elsewhere)
|