|
When Horsley and Clarke invented the technique of controlled
insertion of an electrode into the brain of an experimental animal in
1906, they also invented the term stereotaxic to define it. The name they
chose, stereotaxic, is derived from the Greek stereos meaning solid or
three-dimensional and taxis meaning an arrangement (as in taxonomy). It was argued by some that
stereotaxic should be the proper spelling because Horsley and Clarke had
used it. It was thought, however, that "a three dimensional
arrangement" did not adequately describe the field. Stereotactic
surgery was proposed, being derived from the Greek stereos, for
"three-dimensional," and tactus, from the Latin, meaning,
"to touch". The field involved not only identifying a target in
three-dimensional coordinates, but also actually touching the target with
a probe, electrode, or surgical instrument. The technique they described involves
localizing a target in space, and, in their proposal, the anatomical
structure that lay at that point in space.
It is a three-dimensional concept based on the Cartesian
coordinate system, which states that one point and only one point in
space can be defined by its relationship to three planes intersecting
each other at right angles. The point can be defined by three numbers,
indicating distances from those three planes (anteroposterior, lateral,
and vertical). For their experiments with monkeys (and later other
animals), they proposed the following: the basal plane would pass through
the external auditory canals and the inferior orbital rims (it takes at
least three points to define the location of a plane) or a plane parallel
to that; the midsagittal plane would pass through the midline at right
angles to the basal plane; and the coronal plane would pass through the
external auditory canals at right angles to the first two planes.
Thus, a target point could be defined as follows:
Anteroposterior - mms anterior or posterior to
the coronal plane;
Lateral
- mms lateral to the midsagittal plane; and
Vertical
- mms above or below the basal plane
Stereotactic biopsy:.
The primary purpose of any brain tumor surgery is to find
out what kind of tumor there is in the brain. One of the simplest and easiest ways
is to biopsy the tumor by passing a special needle into the tumor, which
can take a piece of it. In the
brain, however, needles must be guided to the tumor by using the
neuro-image from a CT or MRI scan.
These scans provide computer data, which can be used in the
operating room to guide a needle into the mass. Before this can be done, a reference
frame needs to be applied to the head so that the three-dimensional
coordinate system used in the scanner will be the same in the operating
room.
|
This is the
stereotactic device.
First, a base ring is fitted to the skull using sterile
technique and local anesthesia.
Usually the patient is given some sedation as well. A localizing ring is then attached to
the base ring and a scan is obtained.
The localizing ring has a series of rods, which are arranged in
such a way that they can be seen with the CT, or MRI scans for each
slice. The computer uses the
location of these rods to place each individual scan slice
precisely in three-dimensional space.
In the operating room, the localizing ring is removed and
the arc.ring is attached to the base ring. The arc ring has a moveable guide
tube for
the biopsy needle, which can be adjusted to the exact
trajectory calculated by the operating room computer to approach the
tumor.
A small incision is made in the scalp under local
anesthesia and a small hole is made in the skull with a drill. The needle is passed through the
guide tube to a pre-measured depth, and biopsy samples are
obtained. Once the biopsies are
obtained, the scalp incision is closed and the patient is returned to
their room. Most patients will go home the following day after a
period of observation to ensure that there is no significant
post-operative bleeding.
The benefits of a stereotactic biopsy are that a diagnosis
can be made with a relatively small operation.
The main limitation of the procedure is that the tumor
remains.
The risks of the surgery include the general risks that
exist with any operation which include the risks of infection,
bleeding, anesthesia complications and medical complications.
Risks that are specific to the operation are primarily
related to the risk of bleeding.
If there is significant bleeding, there is a risk of a stroke
and a major operation might be required to remove the blood
clot. The risk of significant
bleeding is about 1 to 2%.
Stereotactic craniotomy:
Stereotactic craniotomy is performed where excision rather
than biopsy of a lesion is planned. Stereotactic localization for
craniotomy is important in small superficial cortical or subcortical
lesions or deep lesions that can be easily missed by conventional
means; and also when accurate
localization is crucial to excise tumors in highly eloquent
areas. This procedure is routinely performed under general
anesthesia.
Although MRI may be used, CT is good enough for most
lesions. After applying the head frame and localiser frame. CT scan is
done and image acquisition is completed for choosing appropriate slices
for target selection. Normally for this procedure seven targets
are chosen – lesion center, lateral edge, medial edge, posterior edge,
anterior edge – these five are calculated from the same axial slice,
and the superior edge and inferior edge – these are calculated from
slices showing the upper and lower limits of the lesion.
The calculation of multiple target coordinates
enables a more accurate planning of the craniotomy as well as aiding in
volumetric excision.
The base ring is attached to the Mayfield adaptor and head
is positioned as to be approximately horizontal with care taken to
prevent compression of neck veins. The posterior, anterior, superior
and inferior edges of the lesion are marked out on the skin using the
sterile pointer – this outlines the lesion. Generally the center target
is used to plan the trajectory. Once the outlining is over, the arc is
swung away and craniotomy performed.Before opening the dura, the arc is
swung back and trajectory confirmed.
Further surgery is the standard procedure of tumor
excision.
In deep-seated lesions the sterile pointer may be passed
directly into the target and locked in position. This will act as a
guide to the target, with dissection being carried around the pointer.
There is inevitably some movement of the brain on performing a
craniotomy, even under stereotactic conditions, and this will affect
the accuracy. This can be overcome by passing a fine silicon catheter
into the target through a burr hole, before performing the craniotomy.
Once the excision is complete the rest of the closure is routine.
|
|
|
|
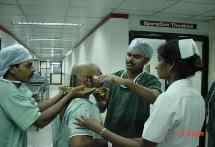
Fixing the base-ring under local Anaesthesia.
|
|
|
|
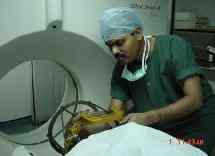
Localiser frame being fitted to the
base-ring on the CT scanner table.
|
|
|
|
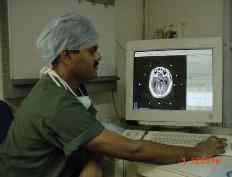
Target localisation and obtaining the
Localiser Co-ordinates from the CT scanner.
|
|
|
|
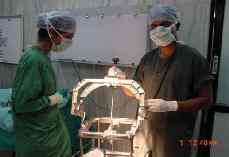
Verifying the
Target Co-ordinates on
the Phantom base.
|
|
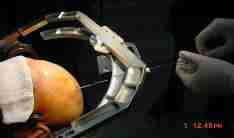
|
|
Arc-localiser
fitted to the base-ring and trajectory chosen,
preparing for biopsy
|
|
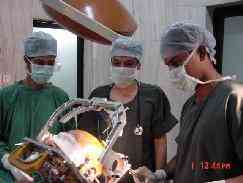
|
|
Biopsy in
Progress.
|
|
Stereotactic aspiration:
|
Aspiration of
deep seated abscesses, acute hematomas, and colloid cysts of the 3rd
ventricle are facilitated by stereotactic applications.
The procedure is similar to stereotactic biopsy.
In case of the colloid cyst, the recurrence is high
and many neurosurgeons prefer microsurgery. However, in selected
cases, this is an effective alternative.
|
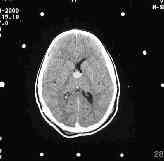 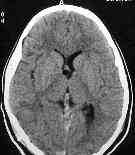
|
|
colloid cyst-pre & post aspiration CT
|
Stereotactic functional surgery:
When radiosurgery was born, stereotactic neurosurgery was
more or less synonymous with functional neurosurgery. Movement disorders
such as Parkinson's disease,
intractable pain due to
cancer, trigeminal neuralgia, and psychological disorders such as
obsessive-compulsive neurosis are the disorders treated.
Some of the commoner procedures, although not yet well
established, are:
Thalamotomies
-to arrest the tremors in Parkinson's disease.
Pallidotomy
-to ameliorate dyskinesia and rigidity of Parkinson's disease.
Thalamotomy or
hypophysectomy
-to relieve intractable cancer pain.
Lesiong of trigeminal root zone at its exit from
brainstem -in primary trigeminal neuralgia.
Bilateral lesioning of the anterior internal
capsule
-in obsessive compulsive neurosis.
It is claimed that the Gamma-knife is more suited for these
procedures.
Test lesioning is not possible and there is
a latent period before the onset of relief.
Recently radiosurgery is being tried for intractable
epilepsy as well.
Stereotactic Radiosurgery (SRS):
|
SRS is a
technique that delivers a dose of high-energy radiation to a targeted
cranial abnormality. Unlike whole brain radiation, X-Knife Stereotactic
Radiosurgery enables precise lesion location and treatment planning
with computer imaging equipment, and then uses precisely guided beams
of focused radiation from a LINAC to treat it.
An X-Knife SRS procedure is completed in one day and the
actual treatment time typically takes less than 30 minutes.
X-Knife produces a radiation dose that results in an effective
treatment of the lesion target,while greatly reducing the dose of
radiation to the surrounding healthy tissue.This non-invasive treatment
avoids the complicationsand inconveniences of open surgery.
|
|
|
Stereotactic
Radiotherapy (SRT):
|
Treatment planning using computer for a
Para-sellar mass lesion.
|
|
SRT accurately delivers lower levels
of focused radiation over a series of treatment sessions called
"multiple fractions." This technique is particularly
important in cases where tumors are adjacent to radiosensitive
tissues such as the brain stem, eyes, or optic nerves, or in cases of
pediatric tumors.
By treating the lesion with lower dose
fractionated therapy, spaced overmultiple sessions, the SRT method
enhances the desired effect on the tumor while reducing the amount of
radiation to nearby critical structures.
An X-Knife procedure is a team effort.
It requires a neurosurgeon, radiation oncologist, physicist,
dosimetrist, and radiation technician. The neurosurgeon fixes the
head frame to the patient. The head frame will remain on the patient
for the entire procedure; it provides a reference for the location of
the patient's anatomy and
|

|
|
tumor during
imaging. It will also serve to immobilize the patient during treatment.
After the frame is in place, a series of CT and/or MR scans are taken.
|
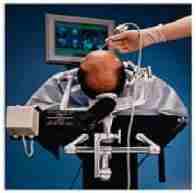
LINAC based
Radiosurgery in Progress.
|
Depending on the type of lesion, angiographic films may also
be ordered.
After the scan is taken, the imaging data is transferred to
the X-Knife computer system. The treatment planning begins by using the
imaging data to produce a 3-dimensional model of the tumor and nearby
critical anatomical structures, such as the optic nerves or brain stem.
The computer system determines the precise target position, dosage and
configuration of radiation beams. This positioning optimizes the dose to
the tumor, while minimizing the exposure of healthy tissue. Once the
physician approves the treatment plan, the LINAC undergoes a series of
quality assurance checks, and then treatment can begin. The patient is
moved into the X-Knife LINAC Suite and is positioned. The actual dose
administration can take as little as 30 minutes. This involves the
movement of the LINAC around the patient as the focused radiation beams
converge on the target. After the treatment, the head frame is removed.
If no complications are observed, patients are free to leave the hospital
the same afternoon.
The indications have increased dramatically. The following
therapies have been reported:
A. Benign Non-Invasive Tumors: When small and
radiographically distinct, radiosurgery can be curative: pituitary
adenomas, acoustic neuromas, meningiomas, etc.
B. Small, Solitary Metastases.
C. Arterovenous Malformations (AVMs): The technique is very
effective for small and medium sized AVMs in eloquent areas or when age
dictates against conventional craniotomy.
D. Adjunct or Boost Therapy: To treat identifiable residual
tumor not removed during surgery, or to augment conventional radiotherapy.
E. Salvage Therapy: To treat inoperable benign or malignant
tumors in patients who have previously been irradiated?
Frameless stereotaxy:
The application of stereotactic techniques to the surgical
resection of brain tumors provides information that allows the use of
minimal craniotomies, accurately localizes subcortical lesions, and may
assist in determining lesion boundaries. As such, stereotaxy-assisted
craniotomy may reduce wound and neurological morbidity and increase the
extent of tumor resection over conventional methods. However,
craniotomies using commercially available stereotactic head frames can be
logistically cumbersome, and techniques that provide information about
tumor boundary demarcation may be either tedious or costly. The
development of frameless stereotactic techniques depended on the
development of the improved spatial fidelity of neuroimagers and the
availability of graphics computers at reasonable costs.
|
Frameless
stereotactic techniques promise to overcome many of these shortcomings
while providing real-time localizing information throughout the
craniotomy. The stereotactic microscope, as developed by Friets et al.
and Roberts et al., and Watanabe et al.’s neuronavigator arm were
pioneering efforts in this area.
The wand tip and trajectory are determined by proprietary
computer software. Real-time display of this information is presented
in multiple, two-dimensional or three-dimensional
displays. When possible, patients were positioned with the anticipated
surgical trajectory nearly vertical. Although not
strictly necessary, this orientation optimizes the accuracy of the
wand, prevents so-called "line-of-site" error, provides a
comfortable working position, and minimizes the effects of "brain
shift" after opening the dura. The present location of the
table-mounted detector array precludes the use of an overhead sterile
instrument table, but draping the patient is otherwise routine .The
localizing wand is used to assist the determination of the lesion
boundary. When visuotactile
information suggested tumor edge, the wand proved confirmatory.
Commonly, in low-grade or deep portions of malignant
astrocytomas (newly diagnosed or recurrent), the boundary was not
apparent and the wand provided guidance that was equal to and more
intuitive than cross- sectional information
provided by the stereotactic frame system.
As in the performance of volumetric resections with frame
systems, it was confirmed that the tumor should be removed in a near en
bloc fashion to minimize otherwise unpredictable distortions
in the spatial fidelity of the tumor/brain interface.
Although brain shift occurs, careful
patient positioning limited this largely to a vertical axis that was
readily detectable on the triplanar display and easily compensated once
it was recognized.
An Optical Tracking System(OTS) procedure begins with the
placement of a number of small, donut-shaped stickers, called fiducial
markers; on the patient's head prior to taking a CT or MR scan. These
markers appear in the scan images and will be used later to match the
patient's anatomy to the CT/MR images in the operating room.
The scan is transferred to the OTS computer, and the OTS
then reconstructs the scan images onto the computer screen as
both two-dimensional and three-dimensional views. The surgeon can now
conduct a review of all scan images from many angles, and determine the
optimal point of entry and trajectory to remove the tumor.
|
|
|
|
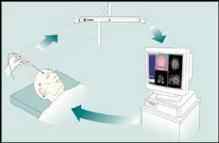
Frameless Stereotaxy – the viewing wand and
the computer work-station – for placement of scalp incision.
|
|
|
|
Frameless
Stereotaxy – the wand, the fiducial, the optical tracking device and
the computer work-station are seen
|
|
|
|
The author with Prof Connolly and Dr.
M.F.Pell, in a frameless stereotaxy procedure, at the St. Vincent’s
Hospital, Sydney.
|
|
The next step is to "register" the patient to his
or her scan images so they can be used interactively during surgery. This
important step is accomplished by just touching each of the markers on
the patient's head with a special probe. This probe can be
"seen" by the OTS camera, and the camera relays the position of
the probe back to the computer.
This relationship allows the computer to know the position
of the instrument in and around the patient anatomy at all times. When
the instrument is placed on the patient, the exact location of the
instrument tip is displayed on the same location on the scan images on
the computer. This enables the surgeon to see the exact location of the
anatomy, which may be obscured by blood and other obstructive tissue.
The OTS offers numerous unique features the
Pointer-as-a-Mouse feature, which allows the surgeon full control of the
software from the sterile field; the Depth Probe, or "virtual
probe", which provides the surgeon the ability to simulate passage
through patient anatomy and visualize critical anatomical structures
before making an incision; and the Universal Instrument Registration
feature, which allows the surgeon to quickly register and then track
virtually any tool during surgery.
With the Optical Tracking System, the neurosurgeon can plan
the most optimal approach to remove the tumor, as well as perform a
smaller craniotomy. This means shorter surgery, reduced recovery time,
and shorter hospital stay.
|