|
Metastasis to the brain is a frequent
complication of many systemic cancers, arising in 10 to 40% of patients
with cancer. The incidence of is increasing due to better control of the
primary disease and thus extending patient survival and allowing
malignant cells time to infiltrate the central nervous system.
Neurological manifestations of systemic
malignancy may be due to metastases, or infiltration neuropathies or
indirect effects, such as metabolic encephalopathy, paraneoplastic
syndromes.
Metastatic complications which can
be further categorized according to location of the lesion; the cerebral
parenchyma, the spine, the skull or skull base, the leptomeningeal space,
and the peripheral nerves. Spinal and skull
metastases are discussed in different sections.
1) Intraparenchymal metastasis:
The brain is a privileged site of
systemic cancer metastasis. Virtually any malignancy can give rise to
intraparenchymal metastases which are the most common intracranial
manifestation of systemic cancer. In adults, the most common sources of
metastatic lesions to the brain include the lung (50–60%), breast
(15–20%), skin (5–10%), and gastrointestinal tract (4–6%). Neoplasms of
the reticuloendothelial system, such as, Hodgkin's, and Leukemia, are
also frequent sources. Various types of sarcomas also may be the source.
Rare cases of metastases from Glioblastoma of the spinal cord seeding
upwards into the cranial cavity along the CSF pathways have been
reported. The age incidence depends on the primary tumor, but the
highest incidence is in the sixth decade of life. The incidence of brain
metastases is lower in children; about 6% in children affected by cancer
and the most common primary tumors are neurblastoma, rhabdomyosarcoma and
Wilms’ tumor. .
Hematogenous spread of malignancy is the
usual mechanism of metastasis. However, direct extension may occur
in the carcinomas of the nasopharynx or breast and other tumors when they
first metastasize to the skull. Epidural metastases result from direct
spread from bones, while subdural plaques are commonly due to hematogenous
spread. On rare occasions, the tumor cells could make their way to the
brain via freely communicating vertebral veins. It is the least frequent
route of spread to the brain.
Metastatic tumors are more often
multiple than single. The designation “single” implies that only one
brain metastasis is present and makes no reference to cancer that neither
may or may nor exist elsewhere in the body. The term “solitary” implies
the presence of a single brain metastasis in the patient who has no known
systemic malignancy. “Multiple” describes the condition in which the
patient harbors more than one intraparenchymal lesion. The histology
is similar to that of systemic metastases.
Metastases from the periphery to the
brain are driven by molecular events that tie the original site of
disease to the distant host tissue. This preference includes such
critical steps as angiogenesis and the preparation of the premetastatic
niche. It appears that the connection between brain and cancer cells is
made in advance of any metastatic breach of the blood–brain barrier.
These chemotactic factors derived from the target or tumor cells may play
a part in lung and breast cancers and malignant melanoma, as opposed to
genitourinary cancers (prostate and ovary) to have a predilection to
metastasize to the brain.
Systemic malignancy can metastasize to
any location in the brain but most commonly affects the cerebral
hemispheres; they are characteristically located in “watershed areas”,
suggesting that microemboli lodge in the capillaries of the most distal
parts of the superficial arteries; this accounts for the tendency of
metastases to be located at the gray-white junction. 80% of brain
metastases occur in the cerebral hemispheres, 15% in the cerebellum, and
5% in the brainstem The distribution of metastases among cerebrum,
cerebellum and brain stem corresponds roughly to the blood supply and
weight of these subdivisions. However, when the respective proportion of
the brain in each of these is considered, metastases are evenly distributed
between the supra- and infratentorial compartments. Metastatic lesions
may also occur to the pineal gland, pituitary gland, and choroid plexus.
Clinical features:
In the majority, the interval between
diagnosis of a primary tumor and that of a brain metastasis is less than
1 year. This interval depends on the primary tumor. It is generally
short in lung cancer or renal tumors but can be several years in the
cases of breast cancer, sarcoma, gastrointestinal or prostate cancer.
Symptoms usually begin sub-acutely. Like
any other mass lesion, intraparenchymal metastases cause symptoms by the
local effects of cerebral tissue compression or invasion. In
addition, an increase in intracranial pressure secondary to the mass
effect of the tumor can give rise to symptoms such as headache, nausea,
and vomiting as a result of edema or compression of the surrounding brain
and may be reversed by therapy. Melanoma, choriocarcinoma, and lung and
renal cell carcinoma are the most likely metastases to have a tendency to
hemorrhage. These lesions may present clinically with sudden onset of
neurologic deficit due to acute hemorrhage and mimic a cerebrovascular
accident..
Diagnosis:
Magnetic resonance imaging (MRI):
Since the introduction of
gadolinium-labelled diethylenetriamine penta-acetic acid (Gd-DTPA), MRI
with its superior anatomic detail, multiplanar capability, and
sensitivity to detection of both intraparenchymal and extra-axial lesions
is the imaging of choice for detection and evaluation of parenchymal
metastases. The typical MRI appearance of a metastasis is of rounded
nodule exhibiting T1 and T2 lengthening. They are associated with a
surrounding area of edema represented by usually differing T1 and T2
lengthening. The cystic or necrotic centre of metastatic tumor, as
it contains highly proteinaceous fluid, is represented by high signal
intensity on T2-weighted images. Sometimes this cystic zone may be
difficult to discriminate from surrounding edema on T2-weighted
images. In such cases differing T1 relaxation rates usually provide
contrast discrimination between central areas of necrosis and surrounding
edema on T1-weighted images.
|
|
|
|
|
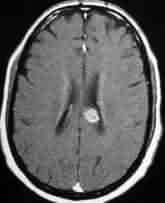
Single metastasis-corpuscallosum
|
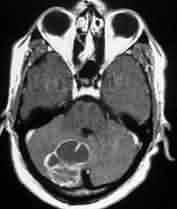
Cerebellar metastases
|
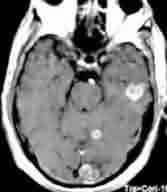
Multiple metastases
|
It is not always possible to distinguish
metastatic deposits from regions of ischemic change, edema,
demyelination, or other benign lesions. SPECT and Magnetic resonance
spectroscopy (MRS) can help.
Computerized tomography (CT):
CT allows detection of contrast-enhanced
lesions as small as 3 to 5 mm. Metastatic tumors are seen usually
at CT scans as discrete, roughly spherical masses surrounded by an area
of extensive edema. Most of them are hypo- or isodense and about 90%
of them show contrast enhancement. In small lesions below 1 cm
enhancement is usually uniform, while the centre of larger lesions often
show irregular or lack of enhancement due to central necrosis. A
ring like peripheral enhancement may mimic an abscess or a malignant
gliom. Acute hemorrhage within and surrounding metastatic tumor may
obscure the presence of the tumor.
Others:
If a brain metastasis is suspected,
systemic diagnostic studies should be performed to identify the primary
cancer. A chest X-ray is indicated to search for a primary or metastatic
lung tumor. A chest CT scan should be performed if the X-ray is negative
and the patient is at risk for primary lung cancer. Female patients
should undergo a mammogram. All patients should have a stool examination
for occult blood. Occasionally, CT scan of the abdomen and pelvis may
detect a primary cancer. Endoscopic study of the GIT may be
needed. Routine peripheral blood picture and a search for tumor
markers such as PSA (prostatic specific antigen) will help. This
facilitates the choice of the optimum management for each individual
patient.
Biopsy:
A biopsy of the intracranial lesion
should be performed in patients with a single enhancing lesion, to
exclude a primary brain tumor, abscess, or other pathology. The
importance of a biopsy in the patient with multiple lesions is less clear
if the patient has a known primary tumor. If there is no known
primary tumor, sterotactic or excisional biopsy is required for a
definitive diagnosis especially in those cases without a detectable
primary.
Management:
The effects of systemic cancer are not
limited to the brain. The majority of patients who have local CNS tumor
control die of extracranial disease progression, whereas those with
uncontrolled brain metastases more often die of neurological causes.
Therefore, achieving local control is of primary importance when
considering treatment options in patients with brain metastases.
Treatment of brain metastases largely relieves symptoms and modestly
improves survival.
Therapeutic approaches to brain
metastases include surgery, whole brain radiotherapy (WBRT),
stereotactic radio-surgery (SRS), and chemotherapy. Many patients are
treated with a combination of these, and treatment decisions must take
into account factors such as patient age, functional status, primary
tumor type, extent of extracranial disease, prior therapies, and number
of intracranial lesions. All of these factors have a role in determining
the overall prognosis and response to treatment.
Symptomatic treatment:
Corticosteroids are recommended in
virtually all patients when brain metastasis is diagnosed because they
rapidly ameliorate symptoms. Steroid administration will decrease
cerebral edema. Which commonly accompanies metastases, but the absolute
need for steroids is dictated by the clinical and radiographic
presentation. Steroids should be administered in the lowest dose
that provides relief and usually are continued throughout the treatment
period, at which time they are tapered.
It is controversial whether prophylactic
anticonvulsants should be administered to patients with brain metastases
who have not experienced seizures. There is no evidence that this
prevents seizures.
There is increasing data to suggest that
medications such as methylphenidate and donepezil can improve cognition,
mood, and quality of life in patients with brain tumors.
Whole-brain radiation therapy (WBRT):
WBRT is recognized as the mainstay of
treatment for most patients with intraparenchymal metastases and widely
available. Radiotherapy is recommended for both radiosensitive and
moderately radiosensitive metastases following surgery. In clinical
practice, WBRT is commonly delivered to patients with multiple brain
metastases not amenable to surgery or SRS, poor functional status, or
active or disseminated systemic disease with effective palliation of
neurological symptoms, and also following surgery. Fractionated therapy
has been shown to
permit more aggressive irradiation
without an unacceptable increase in toxicity.
Nonrandomized studies suggest that WBRT
increases the median survival time by 3-4 months over approximately 1
month without treatment and 2 months with corticosteroids alone. Although
several fractionation schedules have been studied, meta-analyses suggest
that differences in dose, timing, and fractionation do not significantly
alter the median survival times of patients receiving WBRT for brain
metastases. The most common regimen employed is 35 Gy delivered in 2.5-Gy
fractions over 14 treatment days.
The response to radiotherapy depends
upon the radiosensitivity of the metastasis. Lymphoma and testicular and
breast cancers are more radioresponsive than melanoma and renal cell and
colon cancers. Because of the high prevalence of multiple lesions and the
possibility of micrometastases treatment is given to the entire brain.
Multiple attempts have been made to
improve upon the results of WBRT with radiosensitizers have been studied
in randomized controlled trials, all failing to show benefit in either
local brain tumor control or overall survival: lonidamine,
metronidazole, misonidazole, motexafin gadolinium, bromodeoxyuridine and
RSR13 (efiproxiral). Over the years, several chemotherapeutic agents have
been studied in combination with WBRT for patients with brain metastases,
including chloroethylnitrosoureas, tegafur, fotemustine, and teniposide.
More recently, the combination of WBRT and low-dose (75 mg/m2) daily
temozolomide has shown promising response rates with acceptable toxicity
in patients with newly diagnosed brain metastases from a variety of solid
tumors. Current data do not yet support the widespread use of the
combination in patients with new brain metastases.
Addition of SRS to WBRT improves local
control in patients with up to four metastases, it does not affect
overall survival in patients with multiple metastases, and it remains
speculative whether select patients with multiple metastases and
indolent extracranial disease may benefit from SRS boost.
Role of prophylactic cranial irradiation
is controversial. Small cell lung cancer have a >50% estimated 2-year
risk for central nervous system (CNS) relapse. Prophylactic Cranial Irradiation
has been recommended in such patients. There is currently insufficient
evidence to support in other lung cancers.
Surgery:
Indication for Surgical excision must be
individualized. Since the 1980s, resection of most single brain metastases
has become a standard treatment option in patients with good functional
status and controlled or indolent extracranial disease It is strongly
indicated and most beneficial in a single lesion which is surgically
accessible, with low risk of increasing the neurological
deficit. The systemic disease should be under remission or presumed
eradicated. Expected life expectancy after excision must be relatively
long and good quality of life can be expected.
Resection of metastasis offers important
advantages in comparison with other kinds of therapy. It eliminates the
immediate cause of cerebral edema, accomplishes rapid decompression of
the brain and provides samples for histological diagnosis.
Patients with multiple cerebral
metastases do not usually qualify for surgery. It is occasionally
indicated if the patient has one or more small additional lesions in
silent areas of the brain, the systemic disease is under control and
expected quality of life is satisfactory. In such cases excision of
the life threatening or disabling tumor should be undertaken particularly
if the associating lesions are supposed to be radiosensitive or two
metastatic lesions can be removed through the same cranial opening.
Surgical removal of all lesions in selected patients with multiple
cerebral metastases results in significantly increased survival time and
offers prognosis similar to that of patients undergoing surgery for a
single metastasis. Patients with good prognostic features and two to
three metastases may gain similar survival benefit from surgery when the
dominant lesion is resected. The role of surgery is very limited when the
extracranial systemic disease is advanced and in progress. The
extent of systemic disease is the most important variable in
qualification to surgery since the major cause of death is progress of
cancer outside the nervous system.
Stereotactic or ultrasound guidance is
of help to locate small lesions precisely before making cortical
incision. The use of microsurgical techniques allows gentler
handling of tissue, better visualization and control of bleeding.
As complete a resection as possible should be achieved since this is
associated with increased length and quality of survival.
Surgery for recurrence following prior
resection and radiotherapy is advocated when the lesion remains single,
the systemic disease is under control and the general condition of the
patient is satisfactory.
No overall survival benefit is seen with
WBRT after surgery, although patients in the WBRT group are less likely
to die from neurologic causes.
Stereotactic radiosurgery,(SRS):
SRS has emerged as a common treatment
modality for newly diagnosed patients, alone or in combination with
WBRT, and as salvage therapy for progressive intracranial disease after
WBRT. The Cyberknife is used to treat cases in which the brain metastases
are complex in shape or are present in locations that are difficult to
treat using frame-based systems. Gamma Knife, linear accelerator, and
proton beam achieve their effects by treating a discrete tumor with a
high volume of radiation.There is no reported preference in the above
various systems. Choosing a particular SRS system is often based on
institutional, financial, and administrative factors.
Evidence supports the efficacy of radio
surgery in the palliation of symptoms associated with metastases,
providing excellent local control with minimal side effects.
Benefits of radio surgery include its noninvasiveness and ability to be
administered on an outpatient basis, important considerations for those
whose life span is shortened. SRS limited to tissue volumes no greater
than 3 cm in diameter, and the dose administered depends on the tissue
volume. The major drawback of radio surgery is its biological effect of
“radio-necrosis” reported in 4%-6% of patients within 1-2 weeks of
treatment. The fractionated external-beam technique (SRT) allows for
corrections to be made in the treatment regimen as the tumor shrinks, and
for most patients it is easily performed during a short hospital stay.
The use of a small number of fractions supports one of the potential
advantages of radiosurgery (that large fractions are more effective at
killing radioresistant tumors) while adding to the advantages of
fractionation. Stereotactic radiosurgery appears to be a reasonable
treatment option in patients with up to three metastatic lesions in
selected patients regardless of extracranial disease status. In patients
with four or more metastases, SRS should be reserved for those with no extracranial
disease.
SRS or surgery for single metastasis is
debatable. It is generally accepted that conventional surgery is superior
to radio surgery in the treatment of brain metastases. response rates are
mixed for tumors that have traditionally been considered
"radioresistant," for example, renal cell carcinoma, melanoma,
and sarcoma, with some studies showing comparable response rates.
One-year actuarial local control rates in the range of 71 %-79% have been
reported with the use of SRS alone for single and multiple brain
metastases.
Controversy exists over whether SRS
alone is sufficient. The rationale for withholding WBRT is to spare
patients the risk for late neurotoxicity from WBRT. Omission of WBRT in
patients with new brain metastases results in significantly worse local
control and distant intracranial disease control, though it does not
appear to affect overall survival.
The use of SRS for recurrent brain
metastases after WBRT has been investigated in several small series and
appears to be an effective treatment in patients with good functional
status and controlled or indolent extracranial disease.
Chemotherapy:
The role of chemotherapy in patients
with cerebral metastases is limited at present and reserved for patients
who have failed other treatment modalities or for diseases known to be
"chemosensitive," such as lymphoma, small sell lung cancer,
germ-cell tumors, and, to a lesser degree, breast cancer. Methods are
available to reduce systemic dose, and therefore toxicity whilst
increasing tumor dose. These include intra-arterial infusions,
intrathecal administration or even direct placement of drug into tumor.
Drugs may be modified to allow them to pass the BBB, or agents used to
open the BBB. However, because brain metastases are known to have local
BBB breakdown, studies showing roughly equivalent intracranial and
extracranial response rates to chemotherapeutic agents assumed to have
little BBB penetration, particularly when first-line agents for the
systemic cancer are chosen
Prognosis:
The prognosis for most patients with
intraparenchymal metastasis is poor despite the best current treatment
with corticosteroids, radiation therapy, and surgery; however. The
prognosis for patients with cerebral metastases is poor generally in
spite of the fact that there are reports of long survivals. Systemic
disease status is the single most reliable predictor of survival.
Favorable factors influencing survival
in patients undergoing surgery and radiation therapy for cerebral
metastases are
§ lack of
identifiable disease outside the central nervous system,
§ minimal
neurological deficit prior to surgery,
§ long interval
between diagnosis of primary neoplasm and that of the brain metastasis,
§ younger age,
§ controlled or
successfully treated primary disease.
Postsurgical survival time differs
greatly according to the type of primary cancer. In the most
frequent cerebral metastases to the brain from the lung and breast the
median survival time is 11-12months. Prognosis in patients with multiple
metastases and/or progressive systemic cancer is much worse.
Despite surgery and WBRT, 31% to 48% of
patients will develop recurrent metastases in the brain. Therapies
available at recurrence include resection, external beam radiation, radio
surgery, and chemotherapy. The fact that the primary tumor was
responsive to a specific agent or combination of agents does not predict
that the metastasis will respond similarly. Drug delivery is also an
important factor in tumor response as some chemotherapeutic agents do not
penetrate the blood-brain barrier opening.
2) Dural metastases:
Metastases to the epidural or subdural
surfaces of the cranial vault, collectively considered “dural metastases,
are found in 8%–9% of patients with advanced systemic cancer and arise
by either direct extension from skull metastases or hematogeneous spread.
Diffuse studding of dura was seen primarily with breast and prostate canSmall
surgical series. particularly in patients with bone metastases to the
skull. Surgery is the most commonly described treatment for dural
metastases, followed bt radiation.
3) Lepto-meningeal
Metastases:
Diffuse or disseminated infiltration via
the subarachnoid space is relatively uncommon with a poor prognosis, more
so than other types of metastasis. Leptomeningeal disease has been
estimated to account for 8 to 10% of intracranial metastatic diseases
Several terms have been used
interchangeably to describe this condition, including carcinomatous
meningitis, neoplastic meningitis, meningeal carcinomatosis, endothelioma
of the meninges, and meningitis carcinomatosa.
Medulloblastoma, ependymoma, and
pineoblastoma are the primary intracranial tumors most frequently
associated with subarachnoid seeding. However, glioblastomas may also
disseminate within the subarachnoid space. Of systemic tumors, melanoma
and lymphoma and leukemia are the most common followed by breast and
lung. Solid tumors that spread to the leptomeninges include breast, small
cell lung cancer, melanoma, genitourinary, head and neck (usually by
direct extension), and adenocarcinoma of unknown primary.
Both hematologic malignancies and solid
tumors spread to the leptomeninges. The involvement of the meninges with
metastatic solid tumors is often associated with parenchymal brain
metastases. Acute lymphocytic leukemia and high-grade non-Hodgkin's
lymphomas often spread to the leptomeninges without detectable brain
involvement. Small cell lung cancer often involves the leptomeninges and
brain while non-small cell lung cancer usually manifests only brain
metastases. Breast cancer and melanoma spread to both sites.
Several hypotheses have been set forth
regarding the mechanism by which systemic cancer invades the
leptomengines, and there is pathological evidence to support each of
these. There may be hematogenous spread to the vessels of the
choroids plexus or meninges, direct extension from an adjacent
parenchymal, or centripetal extension of tumor along perivascular and
perineural lymphatics through vertebral and cranial foraminae. Tumor most
often spreads to the leptomeninges through thin-walled meningeal vessels
with subsequent dissemination of tumor into the subarachnoid space
Cortical deposits rarely give rise to
lepto-meningeal spread; they form fibrous plaques, perhaps, fibrous
scarring prevent free dissemination. Subependymal metastases may reach
the ventricular surface where their spread is not impeded by fibrous
scarring. An important source of dissemination of neoplastic cells are
metastatic deposits in choroid plexuses. Hydrocephalus may manifest due
to occlusion of CSF pathway. Rarely, spinal epidural deposits can extend
along the course of a nerve root into the spinal leptomeninges. Spread
along the perineural lymphatics from a distant foci is controversial.
After invading the meninges, the tumor
spreads along the route of CSF circulation, seeding the subarachnoid
space. Infiltration is heaviest in the basal cisterns, the sylvian
fissures, and the cauda equina. The cranial and spinal roots are almost
invariably involved in the neoplastic process. The invasion may be
nodular or diffuse. The cauda equina tends to be involved abundantly in a
nodular pattern. Diffuse infiltration involves the entire root, from its
entry into the brainstem or spinal cord, to its exit through the dura and
beyond to the full extent of the archnoid sheath, which stops short of
the dorsal root ganglia. Some roots the nerve fibres remain apparently
unaffected, others show loss of myelin, in others the destruction is
complete and is accompanied by Wallerian degeneration.
Clinical features:
The hallmark of the process is the
presence of symptoms and signs involving multiple loci along the neuraxis. As
with intra-parenchymal metastasis, signs of neurological compromise are
more common than symptoms. Symptoms and signs localized to several
different anatomic sites.
Leptomeningeal involvement at the base
of the brain can use a communicating hydrocephalus that produces signs
and symptoms consistent with intracranial hypertension, such as nausea,
vomiting, and papilledema. Spinal cord and/ or root involvement is
characterized by leg weakness, radiculopathy, reflex changes, and bowel
and bladder dysfunction. Cranial nerve involvement usually follows
spinal involvement is suggested by the signs and symptoms of
ophthalmoplegia, facial weakness, altered facial sensation, or diminished
hearing. Cranial and spinal nerve root symptoms can be attributed to
tumor compression or infiltration of the nerves within the subarachnoid
space.
As leptomeningeal carcinomatosis
progresses, old findings worsen while new signs and symptoms
appear. Treatment usually stabilizes, instead of improving, neurological
disability. Therefore, the clinician must make the diagnosis as
early as possible. This is achieved by suspecting leptomeningeal
metastases in the appropriate clinical setting.
Diagnosis:
Diagnosing leptomeningeal carcinomatosis
can be difficult without a high index of suspicion. Neuroimaging
studies of the affected areas should be performed to exclude other
structural causes of the symptoms and signes and to search for signs of
leptomeningeal tumor. Enhanced CT or, preferably, MRI scans can
reveal linear or nodular leptomenigeal enhancement. Superficial
cortical enhancing nodules are virtually pathognomonic of this disease
but are rarely seen. Enhanced spine MRI is preferable to CT
myelography for identifying “sugar coating” of the leptomeninges or small
nodules. Both methods, however, have a high incidence of
false-negative results, and normal findings on neuroimaging do not
exclude the diagnosis of leptomeningeal metastasis. The sensitivity
of contrast-enhanced MR for the detection of leptomeningeal tumor has
been reported from 33% to 78%. Unquestionably, examination of the
cerebrospinal fluid remains the most sensitive modality.
The diagnosis of leptomeningeal
metastases is usually based on the finding of malignant cells in the cerebrospinal
fluid (CSF). Multiple CSF samples may be required to isolate malignant
cells. CSF often shows increased pressure, pleocytosis, elevated protein,
or low glucose. Other chemistry determinations are sometimes abnormal in
patients with leptomeningeal metastases. These include CSF
beta-glucuronidase, beta 2-microglobulin, human chorionic gonadotropin,
CA 125, carcinoembryonic antigen, and lactate dehydrogenase. The
combination of CSF flow cytometry and selective tumor marker analysis has
been used to aid in the diagnosis of leptomeningeal metastases.
Quantification of biochemical markers in
the CSF can be helpful; some of these include B2-glucurondiase,
carcinoembryonic antigen (CEA), lactate dehydrogenase (LDH), and
B2-microglobulin. B-Glucuronidase is commonly elevated in patients with
leptomeinigeal metastases from malignant melanoma or breast or lung
carcinoma can be increase in any fungal or tuberculosis infection of the
meninges. Marker levels usually return to baseline with successful
treatment of leptomeningeal metastases, and thus, recurrence can be
predicted by rising levels.
Serum CEA levels can indicate the
presence of a systemic cancer. Elevated levels in the CSF suggest
leptomeningeal metastases. Newer diagnostic tests use monoclonal
antibodies directed against tumor cell antigens; it is hoped that these
will offer improved sensitivity and specificity.
Management:
Because of the multifocal involvement of
the CNS, treatment must be directed at the entire neuraxis to be
effective. In practice two modalities are employed; radiation and
chemotherapy. Usually, radiation is given focally to the site of maximal
symptomatology or bulk disease because of the myelosuppression caused by
more extensive radiation therapy. Chemotherapy is used to target the
entire CNS and is usually given intrathecally via lumbar puncture of
intraventricularly by use of a ventricular cannula attached to an Ommaya
reservoir. Approximately 50% of those so treated will have
stabilization or improvement of symptoms.
Intraventricular chemotherapy via Ommaya
reservoir is preferred over intrathecal chemotherapy because of its
reliable drug delivery to the subarachnoid space with minimal discomfort
to the patient. The three standard chemotherapeutic agents used. Methotrexate
and thiotepa have similar response rates in the treatment of solid
tumors. Cytosine arabinoside is preferred in those with a
primary hematological malignancy. High-dose systemic chemotherapy is an
alternative to intra-CSF chemotherapy is an alternative to intra-CSF
chemotherapy, especially in patients with hematological malignancies who
have a concurrent systemic relapse.
Radionuclide CSF flow studies should be
performed prior to the administration of intra-CSF chemotherapy, to
identify blocks that impede the flow of drug and predispose to toxicity.
If flow blocks are detected, radiation should be administered to correct
them.
Lumbar punctures should be performed
periodically to analyze the CSF for response to treatment. Despite
palliation of symptoms, the prognosis of patients with leptomeningeal
metastases remains poor; median survival is 4 to 6 months with treatment.
A number of innovative chemotherapy and immunotherapy trials that may
improve treatment results are currently under way.
In addition to treatment directed at the
CNS, optimal treatment for the systemic malignancy should be
undertaken. While fixed neurological deficits may not improve,
local control of tumor can be achieved; many of these patients eventually
die from the primary illness. Accordingly, patients with controlled
or slowly progressive systemic cancer benefit the most from
treatment.
3) Other CNS
manifestations:
Paraneoplastic neurological disorders
(PND):
The paraneoplastic neurologic syndromes
are a diverse group of diseases characterized by the presence of
neurologic dysfunction in the setting of a remote cancer. Almost any
malignant tumour except brain tumors can be the cause. PND can affect
almost any part of the nervous system, and are most commonly associated
with lung cancer (small cell) and gynecologic tumors. They are believed
to be caused by an autoimmune reaction to an "onconeural"
antigen shared by the cancer and the nervous system. The immune reaction
may retard growth of the cancer, but it also damages the nervous system.
PND may be focal such as paraneoplastic
cerebellar degeneration (PCD) or multifocal such as
limbic and brainstem encephalitis with
sensory neuronopathy. The peripheral nervous system is more commonly
involved than the CNS. Mild distal sensorimotor neuropathies are quite
common in patients with cancer and are not necessarily paraneoplastic;
metabolic, nutritional, and treatment related toxicity must be ruled out.
Other differential diagnosis include vasculitides, inflammatory and
granulomatous CNS disorders, and meningeal infiltrations. They are
degenerative. PCD typically presents as a subacute progressive cerebellar
ataxia, both truncal and appendicular, dizziness, nystagmus (rapid
uncontrolled eye movements), difficulty swallowing, loss of muscle tone,
loss of fine motor coordination, slurred speech, memory loss, vision
problems, sleep disturbances, dementia, seizures, sensory loss in the
limbs. It is believed to be due to an autoimmune reaction targeted
against components of the central nervous system (specifically Purkinje
cells and large brain stem nuclei). It is thought to be caused by an
anti-neuronal Antibody known as anti-Yo.
Lambert-Eaton myasthenic syndrome (LEMS)
is a rare autoimmune disorder which affects calcium channels of the
nerve-muscle (neuromuscular) junction. The etiology of LEMS may resemble
myasthenia gravis, but there are substantial differences between the
clinical presentation and pathogenetic features of the two disorders
Neurological disorders, clinically and
pathologically identical to paraneoplastic syndromes, may occur in some
patients without cancer, but paraneoplastic antibodies are not found in
these patients. The diagnosis of a paraneoplastic syndrome is based on
its increased incidence in patients with cancer, the occasional response
of the neurological syndrome to treatment of the underlying cancer, or
the presence of specific autoantibodies.
In general, PNDs of the CNS are
resistant to treatments except in a few isolated cases. Some
paraneoplastic syndromes respond to treatment of the underlying cancer or
to immunosuppression but, for most syndromes, no effective treatment
exists. A few case reports suggest benefit from intravenous immune
globulin when treatment is started within a few weeks of onset. Low
titers of antibody are associated with a better prognosis of the cancer.
PNDs affecting the peripheral nervous system carry better prognosis.
Neuropathies:
Tumor invasion of large nerves is rare.
In most, the infiltration is confined to the epineurium and the fascicles
may be encased in dense growth without being invaded. The nerve fibres
may escape damage altogether or undergo degenerative changes, both in the
form of demyelination and of axonal degeneration. In the minority of
cases the tumor invades the fascicles and spreads along the subperineural
space and its extensions, along the major endoneurial septa, and along
the blood vessels.
Cervical, brachial, and lumbosacral
plexi can be sources of intractable pain in cancer patients. Pain is
produced when these structures are infiltrated by tumor or compressed by
fibrosis after radiation therapy to adjacent structures. Pain tends to be
less prominent in radiation-induced plexopathies than in tumor-related
ones.
Current treatment is symptomatic.
Intractable pain relief poses the major difficulty. The course of illness
is short, medication providing most support and surgical methods are
rarely employed. Radiotherapy offers no benefit.
Metabolic encephalopathy:
Of all of the non metastatic
neurological complications of systemic cancer, metabolic encephalopathy
is the most common. Metabolic encephalopathy is most commonly caused
by administration of opioids for pain control, vital organ failure, fluid
and electrolyte imbalance, or sepsis. Cognitive difficulties, exemplified
by impairment of memory and orientation, also occur early in the course
of encephalopathy. Characteristically, these changes are reversible
if the underlying systemic metabolic abnormality is identified and
treated appropriately. If uncorrected, metabolic disturbances can lead to
stupor and coma.
Cerebrovascular complications:
Cerebrovascular complications
(infarction and intracranial hemorrhage) secondary to the effects of
systemic malignancy are the second most common neuropathological finding
in cancer patients. Cerebral hemorrhage and infarction are equally
frequent in those with systemic malignancy. Coagulation disorders,
CNS metastasis, and treatment-related complications are the most common
causes of stroke. Cerebral intravascular coagulation is a second
common cause of symptomatic cerebral infarction. Occlusion of venous
sinuses, typically the superior sagittal sinus, while sometimes caused by
an overlying metastasis, can also occur as a nonmetastatic complication
of cancer. The nonmetastatic variety is presumably due to a
coagulopathy caused by the tumor or chemotherapy.
Opportunistic infections:
Patients with systemic cancer are
susceptible to infections of the CNS either because of their primary
disease process or as a result of treatment that renders them
immunosuppressed. They are prone to infections caused by fungi (Candida,
Cryptococcus or Aspergillus spp.) viruses, parasites, and certain bacteria,
such as Listeria monocytogenes. Survival is dependent on the correct
diagnosis being made as early as possible so that treatment can be
initiated.
Treatment Complications:
All modalities employed in the treatment
of metastases to the brain have their risks. Most complications
occur from radiation or chemotherapy. Often, such complications
present as a neurological deterioration, which need to be distinguished
from that which is an effect of the primary process. This
distinction is important as the treatments for the two situations are
diametrically opposed.
Radiation necrosis well known. The effects of
radiation on the brain can be seen acutely within hours, subacutely
within days, or chronically months to years after treatment.
Chemotherapy can be neurotoxic, either
as a direct consequence of the chemotherapeutic agent on the brain or its
vessels or as secondary results from toxicity to other organs (i.e., hepatic
encephalopathy) or from coagulation abnormalities.
|