|
Hemangioblastoma
is the most common benign, primary intrinsic tumor of the posterior fossa
in adults and has also been referred to as hemangioma, capillary
hemangioendothelioma, Lindau tumor, and angioreticuloma.
Hughlings
Jackson described the first case, a cerebellar cyst with an angiomatous
tumor in the cyst wall, in 1872. Von Hippel described retinal
hemangioblastoma in 1904. Lindau recognized the association of retinal,
visceral and cerebellar components of Von Hippel-Lindau (VHL) disease
in1926. In 1928, Cushing & Bailey called it hemangioblastoma which is
widely accepted.
Epidemiology:
They
consitute1% of primary intracranial tumors and 7-12% of posterior fossa
tumors. They may occur sporadically or in about 20-30% of cases as a part
of VHL complex which belongs to a group of disorders known as phakomatoses or
neurocutaneous syndromes. 50% of patients with VHL complex have CNS
hemangioblastoma. VHL complex is a familial disorder which has an
autosomal inheritance with variable penetrance and can be passed on by
the affected and the unaffected members. VHL complex is characterized by
single or multiple hemanigioblastomas in the neuroaxis associated with
one or more of the following: retinal hemangioblastoma, renal carcinoma,
renal cysts, pancreatic cysts,cysts and angiomas of the liver, epididymal
cysts and adenomata and phaeochromocytoma. Familial occurrence of
solitary hemangioblastoma has been reported without any stigmata of VHL
disease. There is an association between this disease and rearrangements
involving human chromosome 3p, most commonly deletion mutations at
3p25-p26, dubbes the VHL gene.
The
commonest age at presentation is between the third and fourth decades
with a male preponderance, and younger in VHL complex.
They
may occur concurrently with other intracranial meningioma and acoustic
neuroma or AVM. Supratentorial locations are rare (about 2-8% of all hemangioblastomas)
and are usually soild. They constitute 2% of spinal neoplasms, usually
thoracic or cervical. 60% of them are intramedullary, with associated
syrinx.
Pathology:
It
is almost exclusively a lesion of the cerebellum (85%). Spinal cord (3%)
is the next common site. Rarely, a hemangioblastoma develops in the
medulla oblongata (mainly in VHL) either as a
solitary lesion or in association with a similar cerebellar tumor. Multiple tumors occur only in VHL.
The tumor is benign but local recurrence occurs with incomplete
removal. Metastases are exceptional.
It is a benign, slowly
growing, highly vascular lesion (usually supplied from pia) of uncertain histogenesis (WHO Grade I).
It
is generally believed that they arise from angiogenic cell precursors.
|
Macroscopically, the tumor may
be solid or cystic with a well circumscribed, dark red mass.
There may be multiple cavernous spaces with areas of hemorrhage.
Lipid deposition may give a yellowish color to the tumor which may be
attached to the dura. Tumor does not line the cyst. The
size of the cyst is unrelated to the size of nodule.
Microscopically,
the
tumor is composed of a mesh of vascular spaces lined by plump
endothelial cells (pericytes surrounding blood spaces). The
vascular spaces are separated by numerous stromal or the
interstitial cells (lipid laden polygonal cells with hyperchromatic
nuclei) with prominent reticulin fibre network.
The
stromal cells, origin of which remains controversial, is
neoplastic. They induce the growth through the secretion of
trophic substances, such as, vascular endothelial growth factor(VEGF).
|
|
|
|
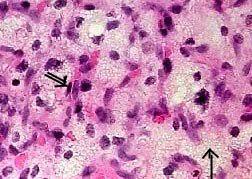
Hemangioblastoma (H&E): Lipid laden
(arrow) stromal cells with thin vascular channels (double
arrow) with prominent endothelial cells and pericytes.
|
|
On immunohistochemistry, the
stromal cells may label with S100, vimentin or even GFAP (which may
suggest a heterogeneous origin for the stromal cells is probably due to
the stromal cells taking up extracellular GFAP protein derived from
adjacent reactive astrocytes), but do
not stain with EMA or cytokeratins.
Clinical
features:
There
are no signs and symptoms specific for hemangioblastoma.
Posterior
fossa lesions usually present with signs of raised ICT with headache
(44%), deteriorating conscious level (84-93%), neck stiffness due to
tonsilar impaction (9%), and pappiledema (70%). 18% of them present with
dementia due to insidious hydrocephalus. Associated cerebellar signs may
be there.
Supratentorial
lesions usually present with focal neurological signs and symptoms
depending upon site.
Rarely,
they may present as SAH or ICH.
Another
rarer presentation is with secondary polycythemia as tumor can produce
erythropoietin.
Spinal
lesions may present with signs of spinal cord compression or syrinx.
Investigations:
Blood
Count
may reveal secondary polycythemia due to erythropoietin production by the
tumor occurs in up to 50% of cases, usually in solid tumors and not in
association with spinal lesions. There is neither splenomegaly nor an
increase in WBCs or platelets. The stromal cell component is responsible
for erythropoietic hormone.
Polycythemia
improves on tumor removal and returns with tumor recurrence and may help
as a tumor marker during follow ups.
|
CT
scan demonstrates
cyst often with isodense mural nodule which uniformly enhances with
contrast. The cyst wall does not enhance.
MRI
is
the imaging of choice. On T1 images, mural nodules stand out against
the darker background of the cyst. The cyst fluid does not appear as
hypotense as CSF. Occasionally, a cyst may show evidence of previous
hemorrhage. Tortuous flow voids suggest vascularity in T2 images. There
is intense enhancement with gadolinium.
Radiological
screening of the whole neuroaxis is recommended, especially in familial
cases.
Differential
diagnosis
include cystic astrocytoma, metastasis (especially renal since
histologically similar and may both be associated with VHL), and
arachnoid cyst if mural nodule too small to see on CT scan.
Supratentorial
lesions may mimic angioblstic meningioma, or hemangiopeicytomia.
|
|
|
|
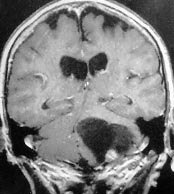
Cystic hemangioblastoma with
a mural nodule-MRI coronal
|
|
AVM
and astrocytoma may mimic spinal hemangioblastoma.
Angiogram
may
demonstrate mural nodule as a vascular mass. Multiple small nodular
lesions can be visualized. Spinal angiogram demonstrates tumor
nodule and vascular supply.
Treatment:
Surgical
removal
of the mural nodule and drainage of the cyst through a appropriate
approach is curative. Cyst drainage alone is of no benefit.
However, solid tumors, including spinal lesions tend to be highly
vascular.
They
should not be biopsied, but an attempt should be made to
remove en masse as for an AVM.
Brainstem
hemangioblstomas also tend to be solid and highly vascular and partial
removal only may be possible. The problems in surgery include risk to the
cardio-respiratory system because these tumors frequently are adherent to
the floor of the fourth ventricle which is close to the
cardio-respiratory control center.
A
peroperative CSF drainage in associated hydrocephalus helps; A shunt, as
a first stage, is recommended by some.
Preoperative
embolisation may be of benefit.
The
location of the tumor is suggested by dilated pial vessels, especially in
solid lesions. Use of an endoscope or stereotactic craniotomy will help
in localizing a small mural nodule which may be missed in conventional
techniques.
Radiotherapy (approx. 50 Gy)
may reduce tumor size and vascularity, but does not prevent regrowth. It
is useful in poor surgical candidates, and technically difficult cases.
The
place of stereotactic radiosurgery for tumors extending into the brainstem
is yet to be evaluated.
Outcome:
Most
series include pre-CT and ‘pre-microsurgery’ cases, so estimates of
morbidity and mortality may vary. This will depend upon the site of the
tumor, its nature (cystic or solid), multiplicity and association with
VHL.
Good
outlook with complete excision of cystic tumor since always benign (90% 5
year survival). With incomplete removal of solid, vascular tumor,
operative mortality can be high (15%) with 50% mortality from tumor
recurrence in survivors.
In
VHL, the survival may depend upon systemic lesions (>25% develop renal
adenocarcinoma). Recurrence rate of 3-10% even after total excision
has been reported. However recurrence may represent a second
primary. Repeated surgeries should be considered. It is recommended
that patient's family should be screened regularly.
|