|
An intracranial aneurysm is a fairly
common incidental finding at postmortem examination, with a prevalence
ranging from 1 to 6 percent among adults in large autopsy series. Many of
these aneurysms, however, are very small, and the prevalence of
incidental intracranial aneurysms among adults undergoing cerebral
angiography is between 0.5 and 1 percent.
Most
intracranial aneurysms remain asymptomatic until they rupture and cause a
subarachnoid hemorrhage.
Some
of them grow to a large size and compress the neighborhood nerves and
present with neuropathies.
Pathology:
Saccular (Berry) aneurysms:
Approximately 85% of all spontaneous
hemorrhages into the subarachnoid space arise from rupture of saccular
aneurysms at the base of the brain. Such aneurysms are not congenital,
but develop during the course of life. Cerebral aneurysms almost never
occur in neonates and they are also rare in children. In those
exceptional cases, there is usually a specific underlying cause for the
aneurysm, such as trauma, infection or connective-tissue disorder.
The majority of intracranial aneurysms
(80 to 85 percent) are located in the anterior circulation, most commonly
at the junction of the internal carotid artery and the posterior
communicating artery, the anterior communicating-artery complex, or the
trifurcation of the middle cerebral artery.
|
Aneurysms of the posterior
circulation are most frequently located at the bifurcation of the
basilar artery or the junction of a vertebral artery and the
ipsilateral posterior inferior cerebellar artery.
Multiple intracranial
aneurysms, usually two or three in number, are found in 20 to 30
percent of patients. In rare cases, as many as 13 intracranial
aneurysms have been detected in a patient.
|
|
Frequency
of
intracranial
aneurysms:
|
|
1.
Internal carotid 38%
2.
Anterior cerebral system 36%
3.
Middle cerebral system 21%
4.
Vertebro basilar system 5%
|
|
|
|
It is largely unknown why only some
adults develop aneurysms at arterial bifurcations and most do not. The
once popular notion of a congenital defect in the muscle layer of the
wall (tunica media) being a weak spot has not been accepted now. Any
defect in the muscle layer is located not at the neck of the aneurysm,
but somewhere in the wall of the aneurysmal sac.
A role of acquired changes in the
arterial wall is likely because hypertension, smoking and alcohol abuse
are risk factors for SAH in general. It may well be the influence of
these factors that leads to local thickening of the intimal layer (
�intimal pads') in the arterial wall, distal and proximal to a branching
site, changes that some investigators regard as the earliest stage in the
formation of aneurysms. The formation of these pads, in which the intimal
layer is inelastic, may cause increased strain in the more elastic
portions of the vessel wall. Also, structural abnormalities in structural
proteins of the extracellular matrix have been identified in the arterial
wall at a distance from the aneurysm itself.
Aneurysms arising from the intracranial
arteries are much more common than those arising from extracranial
arteries of similar size. One possible reason for this discrepancy is
that as compared with their extracranial counterparts, intracranial
arteries have an attenuated tunica media and lack an external elastic
lamina. On microscopical examination, the typical saccular, or berry,
aneurysm has a very thin tunica media or none, and the internal elastic
lamina is either absent or severely fragmented. Thus, the wall of the
aneurysm is generally composed of only intima and adventitia, with
variable amounts of fibrohyaline tissue interposed between these two
layers.
Macroscopically, many intracranial
aneurysms, especially those that rupture, have an irregular appearance,
with one or more daughter sacs and variable wall thickness. The point of
rupture is generally in the dome of the aneurysm.
Genetic Risk factors: Familial
intracranial aneurysms are much more common than has generally been
appreciated. According to several epidemiologic studies, 7 to 20 percent
of patients with aneurismal subarachnoid hemorrhage have a first or
second degree relative with a confirmed intracranial aneurysm. The
familial aggregation of intracranial aneurysms was first described in
1954 by Chambers et al. Considerable evidence supports the role of
genetic factors in the pathogenesis of intracranial aneurysms.
Recent studies have also indicated that
the familial aggregation of intracranial aneurysms is not a matter of
chance. Among first-degree relatives of patients with aneurismal
subarachnoid hemorrhage, the risk of a ruptured intracranial aneurysm is
approximately four times higher than the risk in the general population.
In most families with intracranial
aneurysms, only two or three members are known to be affected, and the
inheritance pattern is unclear. The two main lines of evidence are the
association of intracranial aneurysms with heritable connective-tissue
disorders and their familial occurrence. Of the numerous heritable
connective-tissue disorders that have been associated with intracranial
aneurysms, the most important are autosomal dominant polycystic kidney
disease, Ehlers�Danlos syndrome type IV, neurofibromatosis type 1, and
Marfan's syndrome. It is not known to what extent these specific
heritable disorders are present in the population of patients with
intracranial aneurysms.
As compared with sporadic intracranial
aneurysms, familial aneurysms rupture at an earlier age, may be smaller
when they rupture, and are more often followed by the formation of a new
aneurysm.
Affected siblings are often in the same
decade of life at the time of the rupture.
Environmental
Factors:
Of
the various environmental factors that may confer a predisposition to
aneurismal subarachnoid hemorrhage, cigarette smoking is the only
factor that has consistently been identified in all the populations
studied, and it is also the most easily preventable. The estimated risk
of an aneurismal subarachnoid hemorrhage is approximately 3 to 10 times
higher among smokers than among nonsmokers. In addition, the risk
increases with the number of cigarettes smoked, and patients who continue
to smoke after an initial subarachnoid hemorrhage may be at especially
high risk for the development of a new aneurysm.
It is unclear how cigarette smoking
affects the development of aneurysms, but several hypotheses have been
proposed. Cigarette smoking has been shown to decrease the effectiveness
of  1-antitrypsin,
the main inhibitor of proteolytic enzymes (proteases) such as elastase,
and the imbalance between proteases and antiproteases in smokers may
result in the degradation of a variety of connective tissues, including
the arterial wall. In support of this hypothesis is the observation that
patients with a genetically determined 1-antitrypsin,
the main inhibitor of proteolytic enzymes (proteases) such as elastase,
and the imbalance between proteases and antiproteases in smokers may
result in the degradation of a variety of connective tissues, including
the arterial wall. In support of this hypothesis is the observation that
patients with a genetically determined  1-antitrypsin
deficiency may also be at increased risk for the development of
intracranial aneurysms. 1-antitrypsin
deficiency may also be at increased risk for the development of
intracranial aneurysms.
Hypertension is the most
frequently studied risk factor for the development and rupture of
intracranial aneurysms. Several studies have shown that hypertension is associated
with an increased risk of aneurismal subarachnoid hemorrhage, as well as
unruptured intracranial aneurysms. Although the data are inconsistent,
taken together they suggest that hypertension poses a risk of aneurismal
subarachnoid hemorrhage, but probably not as high a risk as that
associated with cigarette smoking.
The incidence of aneurysmal subarachnoid
hemorrhage, unlike other types of stroke, is higher among women than
among men. Before the fifth decade of life, however, aneurismal subarachnoid
hemorrhage occurs more frequently in men, suggesting the role of hormonal
factors.
The use of low-dose oral contraceptives
by premenopausal women does not increase and may even decrease the
risk of subarachnoid hemorrhage.
The risk of aneurismal subarachnoid
hemorrhage is lower among postmenopausal women receiving hormone
replacement therapy than among postmenopausal women not receiving such
therapy, but not as low as the risk among premenopausal women. These data
suggest that premenopausal women have a low risk of aneurismal
subarachnoid hemorrhage, postmenopausal women have a relatively high
risk, and postmenopausal women receiving hormone-replacement therapy have
an intermediate risk.
A moderate-to-high level of alcohol
consumption is an independent risk factor for aneurismal subarachnoid
hemorrhage. Recent, heavy use of alcohol (binge drinking) in particular
appears to increase the risk of subarachnoid hemorrhage.
The data on hypercholesterolemia
as a risk factor for aneurismal subarachnoid hemorrhage are inconsistent.
Other causes of Aneurysms:
Septic
aneurysms: Infected tissue debris entering the blood stream may
lodge in the wall of cerebral arteries and lead to aneurysmal dilatation.
The traditional term`mycotic aneurysms' refers only to fungi and should
perhaps be discarded; after all, bacterial endocarditis is more common as
an underlying condition than aspergillosis. Aneurysms associated with
infective endocarditis are most often located on distal branches of the
middle cerebral artery, but 10% of these aneurysms develop at more
proximal sites, and rupture of a septic aneurysm causes an intracerebral
hematoma in most patients, but some have a basal pattern of hemorrhage on
CT that is very similar to that of a ruptured saccular aneurysm.
Septic aneurysms in patients with aspergillosis
are usually located on the proximal part of the basilar or carotid
artery. Rupture of such an aneurysm causes a massive SAH in the basal
cisterns, indistinguishable from that of a saccular aneurysm.
Aspergillosis is difficult to diagnose, but should particularly be
suspected in patients undergoing long-term treatment with antibiotics or
immunosuppressive agents.
Severely HIV-infected children may
develop cerebral aneurysms secondary to generalized arteriopathy. In
HIV-infected adults, aneurismal SAH can also be coincidental.
Aneurysms associated intracranial
tumors: Aneurysms associated with tumor are usually incidental. It
is suggested that neoplasm increases local blood flow which
predispose to aneurysms. Some (meningoma) may have dysgenetic factors.
Hormone factors are suggested because of high frequency of pituitary
adenoma with aneurysms.
Cerebral metastases may in exceptional
cases infiltrate the wall of an intracranial artery, and thus cause an
aneurysm to develop, even >1 year after operation on the primary
tumor.
Iatrogenic causes include radiation
therapy, acrylate applied externally for microvascular decompression and
operation for a superficial temporal artery-middle cerebral artery
bypass, with the aneurysm at the site of the anastomosis.
Asymptomatic
Intracranial Aneurysms:
The discrepancy between the prevalence
of incidental intracranial aneurysms at autopsy and the incidence of
aneurismal subarachnoid hemorrhage indicates that most aneurysms never
rupture. With the widespread use of computed tomographic scanning and
magnetic resonance imaging, many unruptured asymptomatic intracranial
aneurysms can now be detected.
The natural history of such aneurysms is
incompletely understood, but all the large studies have reported annual
rupture rates of 0.5 to 2 percent.
The rate of rupture increases with the
size of the aneurysm but appears to be unrelated to the age or sex of the
patient or to the presence or absence of hypertension. Data suggest that
only intracranial aneurysms that are 10 mm or larger in diameter carry a
significant risk of subsequent rupture, but there is still considerable
controversy about the size below which the risk of rupture is negligible.
Screening
The natural history of asymptomatic
intracranial aneurysms is not well defined, and the benefits of screening
have never been quantified. Screening for asymptomatic intracranial aneurysms
appears to be warranted, because aneurismal subarachnoid hemorrhage has a
dismal prognosis, whereas the treatment of most asymptomatic intracranial
aneurysms is associated with a fairly low rate of morbidity (less than 5
percent) and mortality (less than 2 percent).
Screening has been suggested for
patients at high risk for the development of an aneurysm.
The two groups of patients most commonly
screened are those with a family history of intracranial aneurysms, and
those with autosomal dominant polycystic
kidney disease.
In the absence of any clinical feature
or biologic marker that can identify persons in whom intracranial
aneurysms are most likely to develop, screening is generally recommended
for asymptomatic members of families with two or more affected members.
Although the extent of screening depends on the apparent inheritance
pattern in a particular family, usually only first-degree relatives are
screened. Using such a screening program, Detection rate is about 9
percent with affected family members.
Some investigators have suggested
screening of persons even with only a single affected family member.
However, the absolute lifetime risk of subarachnoid hemorrhage for
persons with one affected first-degree relative is small (1 percent at
the age of 50 and 2 percent at the age of 70), even though they have a
risk of aneurismal rupture that is four times higher than that in the
general population. Screening is therefore not recommended for such
persons.
Approximately 5 to 10 percent of asymptomatic
adults with autosomal dominant polycystic kidney disease who undergo
screening are found to have saccular intracranial aneurysms. Clustering
of intracranial aneurysms has been reported in several families with
autosomal dominant polycystic kidney disease, and screening reveals
asymptomatic aneurysms in 20 or 25 percent of the members of such
families. Therefore, although screening for asymptomatic intracranial
aneurysms in patients with autosomal dominant polycystic kidney disease
remains controversial, most investigators agree that screening is
indicated for those patients who also have family histories of
intracranial aneurysms.
Surgery:
Dott successfully wrapped a
ruptured berry aneurysm in 1931. Dandy was the first to use a metal clip in
1944. Since then great strides have been made. The aim of the surgical
intervention is to prevent a rebleed unless it is for hydrocephalus or
intracerebral hematoma.
Pre-operative assessment:
1) Duration since last bleed:
About 40% patients with ruptured aneurysms die following the first
hemorrhage.40% of survivors will rebleed in the I year.25% will rebleed
in 2 weeks, with the incidence markedly decreasing over the next 6wks.
Beyond this, the rebleed rate is about 3% and death is about 2% per year The
proponents of early operation (within 3 days) believe that
overall mortality can be improved by correction of vasospasm by
means of induced vascular hypertension and cerebral perfusion in
the presence of a secured aneurysms. They also claim, irritating
blood products from the basal cisterns, which are presumed to be the
cause for vasospasm can be removed during surgery. Others feel
operative manipulation offset the overall management.
Certainly it should not be delayed beyond the period of vasospasm.
2) Clinical grades & dynamic trend:
If the trend is toward a poorer grade, many believe the surgery should be
delayed. If the trend is towards a better grade, surgery may be
considered.
3) Age:
Generally patients above 60 years do not tolerate major cerebrovascular
procedures as well as the younger do. However each patient should be
considered with her or his prebleed life style and other factors.
4) Associated medical problems:
The systemic problems, such
as diabetes must be corrected before surgery to a reasonable level.
5) Blood pressure:
Rise in blood pressure, assumed due to elevated catecholamines, is usual
in SAH. A relentless increase may herald vasospasm and should alert the
surgeon to possible operative complications Reasonable stabilization
is advised before surgery. A stable blood pressure even on the higher
side is preferable to unstable B.P.
6) Study of the angiography:
Discussion with the radiologist helps, as also presence of the surgeon
during angiography. The site, size, walls, configuration, the number
aneurysms must be studied which will help the surgeon in deciding
the optimal approach.
Length of supraclinoid carotid gives a clue on required frontal
lobe retraction at surgery. State of cross circulations, asymmetry
of circle of Willis, anomalous anterior cerebral artery, carotid
basilar anastamosis, aplasia of one carotid or vertebral must be
studied.
In the absence of ophthalmic artery, meningo orbital artery
(superior orbital branch of middle meningial artery) may be the
primary blood supply to retina and warn the surgeon while drilling
the lesser wing at surgery.
7) Local physiological & anatomical assessment:
Local pathological
changes clot, vasospasm, edema & hydrocephalus can be studied
with CT and or MRI along with angio. When hydrocephalus (10%) or hematoma
(10%)requires surgical intervention to save life, it is prudent that the
aneurysms should be secured at the same sitting.
8) Associated conditions:
a) Reports suggest that 1-2%
of aneurysms are associated with AVM and 5% of AVM are associated with
aneurysm. Ideally both must be treated in one sitting which is not
possible always. Generally, aneurysms is the assumed culprit and
treated first.
b) Carotid stenosis (associated with aneurysms) may be treated first
if the aneurysm has not bleed. In the presence of bleed, aneurysms
gets the priority. Of course, ideal will be if both can be treated
simultaneously.
c) In Moyamoya disease, the aneurysms represent a false one
and may disappear without surgery.
d) Aneurysms associated with tumor are usually incidental. It
is suggested that neoplasm increases local blood flow which
predispose to aneurysms. Some (meningoma) may have dysgenetic factors.
Hormone factors are suggested because of high frequency of pituitary
adenoma with aneurysms. Treatment is directed to symptomatic tumor.
e) Aortic stenosis and polycystic kidney are the only 2
congenital anomalies with correlation with aneurysms. They may have
congenital origin, but they also cause high BP which may be a factor.
f) Connective tissue diseases such as fibromuscular dysplasia, Ehler
Danlos syndrome, Marfan's, Lupus erythematosis, have sporadic association
Ehler Danlos lack collagen and the adventitia and elastic
are ineffective.
g) Familial aneurysms and need to screen the family members are still
unanswered.
h) Aneurysms detected during pregnancy are treated as any other.
i) Mycotic aneurysms carries higher (80%) mortality because of
their fragility.
CT-documented rebleeds have been
reported. Septic aneurysms can be obliterated by surgical or endovascular
treatment, but they usually resolve after adequate antibiotic therapy.
j) Aneurysms in cancer patients are due to oncotic emboli,
destroying the wall of the artery. Cancer gets the priority in
treatment.
k) Traumatic ones are fragile and normally at distal anterior
cerebral and intracavernous int. carotid and need surgical
intervention.
9) Special Tests:
Special tests such as PET, Isotope Scan, Doppler provide information on
CBF and during temporary occlusion.
10) In case of multiple aneurysms:
Ideally all the aneurysms on
one side are attended to simultaneously.
If it is not possible,
the aneurysms which have bled, shall get the priority.
The following will help to
decide the aneurysms which one has bled ,
a) History and clinical exam.
b) EEG
c) Isotope scan-Rapid flow
suggest perfusion; Static flow suggest infarct.
d) CT & MRI-reveals
midline shift, hemotoma and SAH.
e) Cerebral angiography-from
displacement of vessels due to clot, from contour of the aneurysms with
nipple like protrusion, from seepage of contrast.
Largest, in the absence of
above, is assumed to be the one which has bled.
|
Microsurgical
Anatomy:
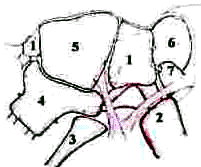
The subarachnoid space:
The regular compartmentalization of the subarachnoid space allows the
surgeon to follow an orderly pattern of dissection. The key landmark is
the junction of the several cisterns which lies above the bifurcation
of ICA just lateral to the optic chiasm.
|
|
|
1. the
carotid cistern (containing the carotid and the origin of its branches)
extends anteriorly to the ant.clinoid.
2. the
sylvian cistern (containing the MCA) extends back into the sylvian
fissure.
3. the
olfactory cistern (containing the olfactory tract) is above on the
base of the frontal lobe.
4. the
lamina terminalis cistern (containing the ACA, the A.COM.A
and their branches) is in the midline.
5. the
chiasmatic cistern (containing the optic nerves, chiasm and
the pituitary stalk) is in the midline.
6.
the interpeduncular cistern ( containing the P.COM arteries and
their branches, the oculomotor nerves, and many components of the
basilar artery circulation) lies beneath the carotid, and chiasmatic
cisterns. The anterior wall reaches from the medial surface of one
temporal lobe to the other.
7.
the crural cistern (containing
the anterior choroidal artery) lies medial to the sylvian cistern.)
|
|
Care must be taken with thickened bands of archnoid which cross
the origins of the middle cerebral and anterior cerebral arteries.
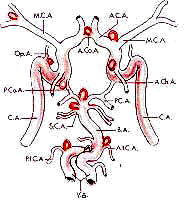
Anterior circulation:
The optic nerve is the landmark in this region. It should be
identified early at exposure.
|
Ophthalmic Artery:
It originates
medially from supraclinoid ICA underneath the ant.clinoid process
(from the subdural portion in 90% and at the carotid-dural ring in 2%
and extradural portion in 8%).It runs along the inferior surface
of the optic nerve, enters the optic canal, penetrates the orbit and
curves medially above or below the nerve.
Superior hypophysial
arteries:
They are multiple arteries from ICA underneath the optic nerve. They
supply the pituitary stalk, ant.pituitary and part of optic nerve and
chiasm and anastomose with counterparts from the opposite ICA
and inferior hypophysial arteries from cavernous ICA.
|
|
Posterior communicating
Artery:
It originates from the
inferior wall of the supraclinoid IAC just distal to the anterior
clinoid process and exits from the carotid cistern , runs medially
(hidden from the surgeon in a subfrontal approach) to enter the
interpedunclar cistern to join the posterior cerebral artery. In 10% of the
patients it is absent or hypoplastic. Its branches supply the optic
chiasm and tract, mammilary bodies, hypothalamus and inferior thalamus.
Anterior Choroidal Artery:
It arises few mms distal to P.com.artery and runs laterally, following
the optic tract posteriorly. It is duplicated in about 30% of patients.
It gives off 'uncal artery' which supplies the uncus, part
of amygdala, and anterior hippocampus. The main trunk supplies the
choroid plexus and anastomoses distally. In its course through the
carotid cistern it supplies, through perforating branches, to
inferior chiasm, parts of optic tract & globus pallidus, the
genu of the internal capsule, part of cerebral peduncle, red
nucleus, the subthalamus and thalamic nuclei and further distally, to
lateral geniculate body, the internal capsule and the optic radiations.
Obviously loss of this vessel is devastating.
Middle Cerebral Artery:
The ICA bifurcates at a variable distance from the anterior clinoid. The
middle cerebral, from the origin to bifurcation is termed the
'M1'segment which gives off the superior lateral and the inferior
medial perforating branches (lenticulo-striate) at its proximal part. The
lenticulo-striate branches supply the anterior commisure, the
putamen, the lateral globus pallidus, the superior internal capsule and
the head and body of the caudate nucleus. The surgeon must look for these
during archnoid dissection. The 'M2'segment is distal to the bifurcation.
In about 20% of the patients there is a trifurcation instead of
bifurcation. The orbitofrontal, pre-frontal, angular and posterior
temporal arteries arise proximally from 'M2' and supply the cortex.
Anterior Cerebral Artery:
Anomalies are common, especially in patients with aneurysms. The 'A1'
segment extends from the carotid bifurcation to the anterior
communicating artery and the rest is termed the 'A2' segment.
Typically one A1 is
dominant. The proximal perforators postero inferiorly and distal
perforators close to the anterior communicating artery, and
perforators from the anterior communicating artery (typically from the
inferior side of the neck of the aneurysm) supply the
infundibulum, optic chiasm, fornix, internal capsule, striatum and
hypothalamus. In 25% of the patients the communicator has various
anomalies.
The recurrent artery of Heubner usually arise close to A.Com.Art. from
A1 or A2 segment and runs parallel to the anterior cerebral artery
laterally along the inferior frontal lobe and at risk during resection of
the rectus gyrus. It supplies parts of caudate nucleus, the putamen,
the globus pallidus,and internal capsule.
Posterior circulation:
Vertebral Artery:
It is the 1st branch of the subclavian and enters the transverse foramen
of C6. At C3 it turns laterally and enters the foramen at C2 and
exits through the foramen of C2 behind atlantoaxial joint and lies
along the posterior arch of C1. It penetrates the dura at foramen
magnum, goes laterally and then ventrally to join the contralateral
artery to form the basilar artery.
In 15%, one artery is dominant.
In addition to the posterior inferior cerebellar artery and
anterior spinal artery, it gives off perforators to the to medulla
and occasional posterior spinal artery and few meningeal branches.
Posterior Inferior Cerebellar Artery:
It is quite variable. In about 10%, it is absent. In about 50% it arises
from the proximal vertebral artery. It loops along the lateral
medulla and turns superiorly to complete the caudal loop. It then
passes superiorly as a cranial loop and then crosses the cerebellar tonsil.
The artery supplies the choroid plexus of the 4th ventricle, cerebellar
tonsil, vermis and cerebellar hemisphere.
Basilar artery:
The vertebral arteries join along the anterior surface of the medulla,
close to the pontomedullary junction. The artery is deviated to the side
of the smaller vertebral artery. It extends along the ventral surface
of the pons and terminates in the interpeduncular cistern into
posterior cerebral arteries. In about 50%, the tip is at the level of the
posterior clinoid. In 15%, the labyrinthine or the internal auditory
artery arises from the basilar artery. The perforating arteries
from the trunk project posteriorly. The anterior cerebellar artery
and superior cerebellar artery are the major branches.
Anterior Inferior cerebellar Artery:
In most, it arises from the proximal basilar. Typically the right
and left arteries arise at the same level. It runs inferiorly and
laterally to the IAM. The labyrinthine artery arises from AICA in about
85% of the patients. It also supplies the pons, middle cerebellar
peduncle, flocculus, tegmentum and cerebellar hemisphere.
Superior Cerebellar Artery:
In 85% of the patients, the left and right ones arise from the basilar as
a single trunk. At its origin, it is separated from the posterior
cerebral artery by the 3rd nerve. It supplies the superior the superior
aspect of the cerebellar hemispheres, the superior cerebellar peduncle,
the dentate nucleus and a portion of the middle cerebellar
peduncle.
Surgical instrumentation:
1) A binocular dissecting microscope with its superb
magnification, clearer stereoscopic images, and improved
illumination is a must, needless to say. It makes it possible for a
smaller exposure with less brain retraction. Micro instruments along
with bipolar forceps, lyla retractors and fine tipped suckers
are essential for fine dissection.
2) Clips: Generally, shorter clips have more closing pressure,
the pressure is more near the shank.
(a) Yasargill's clips are
cross action clips and popular. Their small shank do not obscure
vision.
(b) Sugita's clips are some what similar and comes in various angles.
(c) Heifetz clips have broader wings with an internal spring action and
are preferred for thin, friable walls by some.
(d) less commonly malleable clips are used.
(e) Temporary clips differ from permanent clips with their closing
pressure not exceeding 25-40 gm.
(f) Fenestrated and the right angled ones are ideal for larger aneurysms
with a broad neck, especially at the internal carotid and basilar
tree.
3) Other agents:
Protective coatings include muscle, fascia, gelatin, cotton and
synthetic agents such as methylmethacrylate, EDH adhesive (bioband)
come handy when surgeon is faced with unclippable aneurysm. Cotton
is the only one with proven effect.
Surgical Technique: click for intraoperative videos
|
Anterior
Circulation:
With the exception
of distal arterial aneurysms which are rare, all the anterior
circulation aneurysms can be clipped through a pterional flap of
various sizes. In fact basilar tip aneurysms especially the
ones which project above the level of posterior clinoid can
be successfully clipped trough this approach.
Added orbito
zygomotomy helps in minimizing the brain retraction. Pterion is the
point where zygomatic process of the frontal bone, orbital ridge and
temporal bone meet. Pterional flap is really a modified Dandy's fronto
lateral flap.
Following
craniotomy, the lesser wing of the sphenoid should be shaved down to
ant. clinoid until the meningo orbital branch of the middle meningeal
artery is encountered. On occasions, removing the anterior clinoid helps.
It is a must, for medially projecting internal carotid artery aneurysms
(ophthalmic) to visualize both sides of the neck.
L.P. drainage, as
preferred by some, may be started at this stage (to restrict the brain
retraction) after the dural-pericranial hitch stitches. Others
prefer to open the sylvian fissure and other basal interns and let out
the CSF. L.P. drainage hinders arachnoid dissection and may injure the
olfactory tract.
Opening the sylvian
fissure is harmless, the bridging veins from the frontal lobe to
sylvian vein may be cauterized and cut so that the major vein is
preserved.
Extension of the
neck at this stage further minimizes the brain retraction.
Presence of a
temporal hemtoma may warrant an approach through superior temp
gyrus in middle cerebral artery aneurysm. Some prefer this
approach as a routine to avoid brain retraction although getting a
proximal control (in case of accidental rupture of the aneurysm) is
difficult.
Similarly a small
group of surgeons approach ant. com. art. aneurysm. through an inter
hemispheric approach.
Whatever the
approach may be, fine arachnoid dissection of the neck, without exposing too much of the distal
artery is necessary.
Intermittent use of
temp. clips, not to exceed 10 - 15 mts each time, help
in dissection.
The B.P. should
kept above normal BP during temp.occlusion to maintain adequate
collateral circulation. On occasions, it may be wise to puncture
the aneurysm (preferably with temp clips in place) to facilitate
dissection.
Posterior circulation:
(a) Basilar tip
aneurysm can be approached
through a pterional approach with additional
zygomatico orbitotomy or a sub-temporal approach with or without
temp. lobe resection. Side of the approach depends on the
projection of the dome which is to be avoided.
It is prudent to
avoid dominant, side every thing else being equal.
b) Sub
temp.approach is well suited for post. Cerebral artery aneurysms.
(c) Vertebro
basilar aneurysms below the level of int. auditory meatus are normally
approached through a suboccipital approach. Lately, extended
lateral approach has been recommended basically to gain better exposure
with lesser retraction. The patient in park bench position, a
lateral suboccipital craniectomy is performed.
The arch of the
atlas is removed. The inter transverse process space of C1&2
exposed and posterior root of C2 is cut to expose the vertebral artery
which is protected and the dura is opened medial to the dural
entrance of the vertebral artery. L.P. drainage at this stage helps to
minimize brain retraction. The occipital condyle is shaved to such
an extent that the dural flap falls flat over the shaven
condyle, rather than getting tented up.
(d)
Aneurysms above the level of IAM are better approached
through trans tentorial approach. petrosectomy helps in minimizing
brain retraction.
Lately, a new
approach, the trans petrosal approach where-in a window is made
through the apex of the petrous extra durally, has been described. This
avoids brain retraction and injuries to the cranial nerves which is
almost inevitable in posterior and postero lat. approach.
Whatever the
approach, the basics, such as adequate exposure and fine arachnoid
dissection of the neck before applying the clip, must be adhered to.
The temp.clips may be kept longer (20 - 30mts) in posterior
circulation.
Giant aneurysms
(>2.5cm):
They may be
saccular or fusiform.
|
|
|
|
|
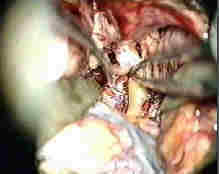
Lt.Ant.clinoid removed,extradurally
|
splitting Rt.Syl.Fissure
|
|
|

|
|
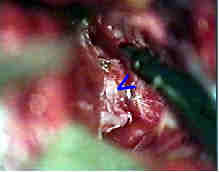
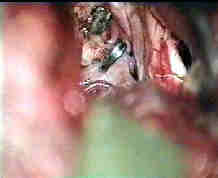
opened Rt. Syl.Fissure
|
Rt.optic nerve& ICA
|
|
|
|
|
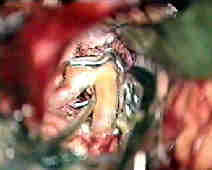
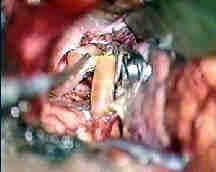
Rt.A.com.art.An
|
....clipped
|
|
|
|
|
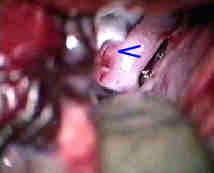
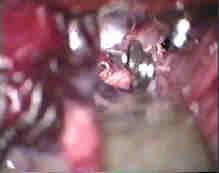
Fenestrated clip for Rt.A.com.An
|
....clipped
|
|
|
|
|
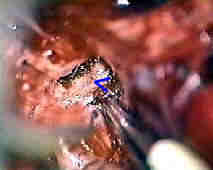
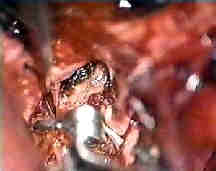
Rt.ant.ch.art.AN
|
....clipped
|
|
|
|
|
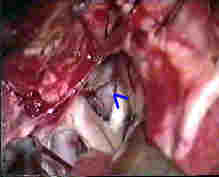
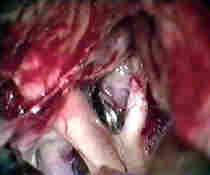
Rt.ICA.An
|
....clipped
|
|
|
|
|
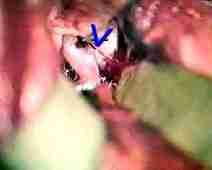
Rt.ophth.art.An
|
....clipped
|
|
|
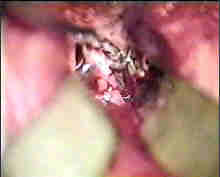
|
|
Rt. P.com. art An
|
....clipped
|
|
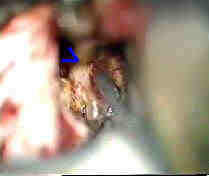
|
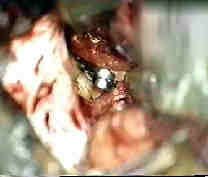
|
|
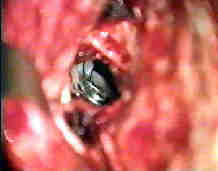
Rt.Bas.tip An-subtemp.app
|
....clipped
|
|
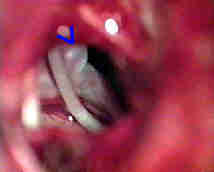
|
|
|
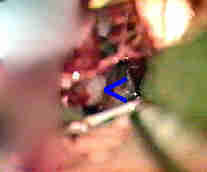
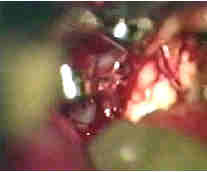
Rt.AICA.An-transpetrous app. with
5th and 6th nerve
|
....clipped
|
|
|
|
|
Rt.Vert.art.An-suboccipital app.
|
....clipped
|
|
(a) Saccular aneurysms must
be considered for clipping these days, whatever the size is, as they are
not ideal for interventional radiology as it stands today. Broad neck, calcification
at the neck, presence of intraluminal thrombus are more common in giant
aneurysms and make clipping a difficult proposition. Angled, fenestrated,
strong and extra strong clips are required. It is unusual that a single
will suffice.
Three basic systems of clip
application are often used.
(1) Piggyback or booster
clip is a second clip placed so as to increase and reinforce the closing
pressure of the primary clip.
(2) Tandem clipping involves application of 2 or more clips across the
neck usually with short blades across the distal neck. With progressive
distal application of these clips, a neck can be fashioned and occluded.
(3) Picket fence or parallel clipping is a system wherein clips
are applied parallel to one another usually perpendicular to the
plane of neck, lined up like a picket fence. It is wise to expose
the proximal artery such as carotid or vertebral before dissection.
Temporary proximal occlusion or aspiration of the sac or the use of
an encircling silk ligature to narrow the neck may make clip occlusion
possible. Rarely hypothermia (down to 16 degree c) and cardiopulmonary by
pass in selected cases may be useful.
(b) Fusiform aneurysms have
no neck and not amenable for clipping. Excision and anastomosis with
graft is ideal. But proximal (Hunterian ligation) or trapping are more
commonly used with or without bypass.
Interventional
radiology has largely replaced the need of these measures. When such
measures are contemplated without bypass, the following pre op tests help
to assess the viability of such procedures.
1) Mata's test - Common carotid is compressed at bed side for 10 mts. and
the pt is examined clinically for a deficit (unreliable
and uncomfortable).
2) Temp. occlusion (at surgery) for 30 mts and the pt is observed with or
without EEG. This reveals only pts who are immediately
intolerant and is of no help in the rest.
3) During angiography a
cross circulation may be assessed with contra-lateral compression. 70% of
those with giant aneurysms will show filling of opposite AC & MC
and are good candidates. 25% of them fill only AC and ligation may be
considered with some risk.
4) Carotid artery pressure determinations are reliable. Both carotids are
exposed and the clamp applied. The pressure of the carotid stump distal
to the clamp is measured. If the reduction of the pressure is not more
than 50% when compared to the unclamped one the procedure is safe.
5) Ideal is Xenon study with temp clamping. If the CBF is more than
40ml/mt/100gm the procedure is safe. Less than 20 ml it is not safe. In
between it is probably safe if the reduction is less than
25%. Crutch field clamp is ideally used.
Post operative care:
Absolute bed rest with head kept flat and close surveillance
of electrolytes is warranted. Some prefer to institute, 'triple
H' (Hypervolemic, hypertensive, hemodilution) treatment for 2-3
days. Anti-convulsants are routinely given as the incidence of post
op fits is next only to that of abscess.
It is wise to repeat angiogram in cases where there is doubt of complete
occlusion. If facilities available an intra operative angiogram may
be arranged. If done, brain swelling must be anticipated.
Whatever discussed above is for some one at the bottom of
the learning curve. As they go up in the curve, they form their
own principles and technique, after all, medicine is an ever
changing field!!.
|