|
Spina bifida is the commonest of neural tube defects.
Spina bifida occulta is much more prevalent than open variety. It is a
failure of fusion of the vertebral arches, most frequently the fifth
lumbar and first sacral, without a protrusion of the cord and meninges.
It may involve only one vertebra or may extend over several segments. It
is seen as an incidental X-ray finding in 17-30 per cent of the normal
population.
For the majority it carries no clinical
significance but an occasional patient may have an occult spinal dysraphism
in the form of a tethered cord, midline spur, lipoma, dermoid or an
abnormal dilatation of the sacral end of the dural sac (occult
intrasacral meningocoele). The overlying skin may be normal or stigmata
of adysraphic state, such as hypertrichosis, haemangioma, skin
pigmentation, dimples, sinuses and "pedunculated tail" may be
present.
They may be collectively called
'Tethered cord syndrome.
Tethered cord syndrome (TCS):
Progressive neurological deterioration
localized to the lower spinal cord resulting from traction on the conus
medullaris has been termed TCS. Tethered cord is associated with low
conus below the mid L2 level and a short, thick filum, attached to the
dura, or an extradural band may anchor the cord. It is the cord which is
tethered, with the nerve roots lying lax and even loosely on either side.
The effects of the tethering is maximum close to the site of tethering
and when the tethering lesion extends over a distance the maximum effect
is on the cord adjacent to the caudal end of the lesion, where the
maximum stress lies.
Pathology:
Any process that tethers the spinal cord
can result in a patient who has tethered cord syndrome. Children can be
born with normal anatomy and develop a tethered cord (secondary) through
an acquired process, such as infection, scarring, or tumor. This section
focuses on congenital (primary) causes of tethered cord.
Lipomyelomeningocoele and a thickened filum terminale accounts for 70
per cent of TCS.
|
Fatty filum is the
simplest form of conditions causing TCS. In this condition, the filum
terminale, can be thickened, potentially with lipomatous tissue.
Hoffman and colleagues have suggested that a diameter of 2 mm or
greater should be considered an abnormally thickened filum. Impaired
canalization of the growing secondary neural tube (the neural cord)
with cells capable of growth and differentiation, particularly
preadipose tissue, is believed to be the cause of both the thickened
and fatty filum terminale. The fatty filum commonly is associated with
cases of imperforate anus, suggesting a common timing of pathogenesis
during development.
Lipomyelomeningoceles are more
extensive lesions and represent a combination of a splayed cord fused with
a lipomatous mass, which in turn fuses with the subcutaneous adipose
tissue. The neurologic deficits of cord tethering are probably caused
by impaired circulation in the stretched cord, as evident by its
reduced oxidative metabolism or to abnormal development as the cause of
the neurologic deficits as in myelomeningocele.
An intradural lipoma has no
anatomic connection with the subcutaneous fat and lies wholly within
the dural space in embryologic terms. This is similar to a
lipomyelomeningocele except that the neural tube closes after
|
|

|
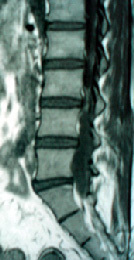
|
|
tethered cord
|
tethering due to lipoma
|
|
the mesenchyme has entered. In some patients,
the cord is bound down by lipomatous tumors or fibrous tissue, the sacral
roots ascending. Lipomas may attach dorsally to the conus and be sessile
or pedunculated, while other lipomas occupy the distal end of the conus,
elongating the latter and terminating in a small lipoma with attached
nerve roots. The dorsal and caudal lipomas may occur in combination.
Filar lipomas occupy an enlarged filum terminale. Lipomas most are
commonly subpial, although a small number can be subdural. Subdural
lipomas are infrequently associated with tethering and more commonly
present like a mass lesion with cord compression. More commonly, however,
lipomas of the spinal cord occur in the lumbosacral region and have an
associated dural defect.
Dorsal dermal sinus is a long,
thin, squamous epithelium-lined sinus that extends from the skin surface
for a variable distance. Like most dysraphic abnormalities the commonest
sits for the defect is the region of the posterior neuropore i.e.the
lumbo-sacral region, the next frequent site being the sub-occipital
region. It may, however, occur anywhere in the dorsal midline from the
nasion to the coccyx. It may rarely be away from the midline. The sinus
may terminate extradurally, intradurally or extend into the fourth ventricle
or the medulla where it may produce an abscess or a dermoid. The
defect arises as a result of failure of separation of the neuroectoderm
from the epithelial or surface ectoderm. This separation normally takes
place between the 3rd-5th week of intrauterine life. The congenital
dermal sinus is mostly superficially located and presents as a dirnple.
This sinus may become symptomatic due to an infection if it has a
connection within the theca, distorts growth of the neural tissues or
compression of neural tissue if associated with a dermoid/epidermoid.
Meningitis is the most common
presentation. The cutaneous opening may at times be inconspicuous with
surrounding hairy growth, naevus, pigmentation or lipoma, When infected, the
opening of the sinus becomes red and inflamed with erythema of the
surrounding skin. A clinical picture of spinal arachnoiditis may be seen
as a result of recurrent attacks of subclinical infection. Any or all the
neurological features observed in spinal dysraphism may be encountered in
this condition. Spinal compression, extradural, intradural or
intramedullary, may be caused by an associated dermoid or epidermoid cyst
or by a secondary localised abscess. The neurological level of the
lesion is usually several segments higher than the external opening, as
the dermal sinus runs an oblique course from the skin in a cephalad
direction.
Split cord malformation (SCM) results
from abnormal splitting of the notochord. This new nomenclature was
introduced by Pang, et al., in 1992, to eliminate confusion created by
the use of the terms diastematomyelia and diplomyelia. Diastematomyelia
usually refers to a split cord in which the two halves are separated by
a bone spicule and contained within separate dural sleeves. In contrast,
the term diplomyelia is generally used to describe a condition of two
hemicords within one dural sac, often with two complete sets of nerve
roots, separated by a fibrous band. Pang, et al., proposed a common
origin of both malformations: an adhesion between the ectoderm and
endoderm leads to an endomesenchymal tract that bisects the spinal cord.
If the tract also contains cells of the precursor cells of the meninges
(meninx primitiva) the resultant malformation would be SCM Type I, or
diastematomyelia. Otherwise, the formation of a separate dural sleeve
and bone septum does not occur, and the malformation is a SCM Type II,
or diplomyelia.
In both SCM I and SCM II, when neural
crest cells are also involved in the split, two sets of dorsal roots will
develop with each hemicord having paramedian dorsal roots as well. These
rootlets may end blindly at the midline septum or go beyond the dura
along with a leash of abnormal vessels and fibrous tissue to form the
fibroneuro vascular stalk of a myelomeningocoele plaque. The
endomesenchymal iract may persist to a variable extent. When it goes upto
the cutaneous ectoderm, it may form a fine dermal sinus or a wide
myelomeningocoele. It may differentiate to form the various cutaneous
stigmata, lipoma, dermoid or epidermoid cysts. In a type ll SCM the
fibrous septum may be entirely ventral to the cord, with the dorsal
surface of the cord looking normal.
The common site of this defect is the
lower thoracic or lumbar region. When the lesion is situated in the
lumbar region the cleft often is through an unusually low placed conus.
Both types of SCMs represent lesions
that tether the spinal cord during growth and movement. The lesion
manifests itself commonly in children, with a higher incidence in
females. Occasionally cases presenting with symptoms in adult life have
been reported. The development of symptoms and signs appears to be
related to periods of rapid skeletal growth. Prevention of the normal
ascent of the cord by the spur during these periods has been blamed for
the development of the neurological deficit. It is the spur and not the
divided cord that is the cause of the symptoms.
A myelocystocele is a variant of
hydromyelic dilatation of the central canal, the cystic cavity being within
the cord and the spinal roots originating at the ventral and dorsal outer
surface of the cyst wall. A myelocystocele is often associated with
defects of the vertebral bodies or intestinal fistula. These types of
lesions are often located in the cervical or upper thoracic cord at the
level the underlying bony defect. They often are associated with a
lipoma (lipomyelocystocele).
Neurenteric cysts often are found
on the ventral side of the spinal canal and consist of a fluid-filled
cyst that may communicate with the gastrointestinal tract through a
vertebral defect such as a hemivertebra or butterfly vertebra. The
neurenteric cyst itself can cause compression, but its adherent fibrous
bands also can result in tethering.They usually are intradural and extramedullary,
and their origin is debated, although positive immunoreactivity for
carcinoembryonic antigen suggests endodermal origins.
Tethering can occur with meningocele and
meningomyelocele, as functional cord attaches itself dorsally either to
dura or to surface ectoderm. An interesting case of meningocele known as
the "meningocele manque" (the "missing" meningocele)
occurs when a meningocele has formed during embryogenesis but has healed
spontaneously or scarred creating a dorsal band. These dorsal bands can
extend from intrathecal structure into the dura or outside structures
creating a significant tethering effect [34]. The dorsal band of
meningocele manque may reflect a fibroneurovascular stalk derived from
the same endomesenchymal tract that is the basis for split cord
malformations.
Anterior meningocoeles are rare
lesions and may occur in the pelvis through a defect in the sacrum, the
meninges may protrude into the pelvis causing compression of the pelvic
organs or the condition may present as a neurogenic bladder. The sac may
harbor benign tumors like a lipoma and the filum terminale attached to
the meningocoele may produce a tethered cord syndrome.
Intrathoracic meningocoeles are often associated with neurofibromatosis
and are frequently confused with mediastinal tumors. However,
meningocoeles cause fewer physical signs than tumors. X-rays of the spine
often show erosion of the posterior aspect of the dorsal vertebrae. These
meningocoeles are best left alone except when there are compelling reasons
forcing surgery.
Clinical Features:
The clinical presentations differ widely
in children and in adults. Rapid early deterioration within the first
weeks after birth characterises those lesions associated with imperforate
anus, omphalocoele and exstrophy. The onset of tethered cord syndrome is
related to the level of the pathology and the height and age of the
patient.
Pain is invariably present in the adult
as a diffuse, dysaesthetic "central'' type of pain over the legs and
perineum and in some patients may mimick a lumbo-sacral intervertebral
disc protrusion. In chidren pain is uncommon feature and is limited to
the lumbosacral region.
A slow onset of difficulty in
micturition or a limping gait in a child seven or eight years old or
more, should arouse the suspicion of a tethered cord.
Cutaneous stigmata of dysraphism are
almost always present in a child while only about half the adults have
them. They include the midline lumbosacral cutaneous hemangiomas,
lumbosacral hypertrichosis, the lumbosacral dermal sinus, the midline lumbosacral
subcutaneous lipoma, and the lumbosacral skin appendage. The cutaneous
stigmata are present in approximately 50%70 of patients who present with
TCS.
The hallmark of the neurological
deficits is their asymmetry. Sensory, motor and bladder involvement are
frequently seen in children as well as adults. Neurological
deterioration with both upper and lower motor neuron involvement takes
months or years and symptoms appear during late childhood. In children
motor involvement is in the form of gait disturbance and in the adult
there is actual weakness and wasting.
Bladder symptoms in the form of
incontinence or repeated bouts of urinary tract infection may be the only
symptom. Orthopedic deformities such as scoliosis or kyphoscoliosis is
common. There may also be deformities of the feet, e.g., pes cavus,
talipes equinovarus or trophic skin lesions. They are in adults.
Imaging:
Plain roentgenograms are almost always
abnormal. There is usually a spina bifida, a circumscribed median bony
opacity, a widening of the interpedicular distance or an associated
hemi-or block vertebra.
MRI is well suited to identifying the
level of the conus relative to vertebral bodies, the presence of a
syrinx, or visualization of other pathologic processes. MRI also is able
to define the anatomy of other causes of tethered cord, such as the
anatomy of terminal or multiple lipomas, the presence of congenital
lesions (such as dermoids), and the presence of myelomeningocele. Its
limitation, however, is the poor quality of images in infants, the
difficulty in interpreting the various aspects of a complex malformation,
and its inability to define bony ventral abnormalities like the bony
septum in a split cord malformation.
Ultrasonography can have a role as
a relatively quick and easy screening tool in young children.
Surgical Treatment:
Development or progression of symptoms
in patients who have a tethered cord often calls for an untethering
operation. Patients who have large spinal lipomas that exhibit mass
effect on the spinal cord, and presenting only with pain may be
considered for a trial of weight loss before committing to surgical
intervention.
A group of myelomeningocele patients who
have symptoms suggestive of tethered cord syndrome and who also have
ventricular shunts. A malfunctioning shunt sometimes can cause signs and
symptoms that may mimic a tethered cord, and shunt correction
helps. Orthopedic deformities may be the presenting symptoms,
and untethering should be considered before orthopedic correction. data
suggest that the neurosurgeon should recommend untethering as treatment
of the root cause of scoliosis in selected cases, but that correction of
the deformity may be limited, and orthopedic involvement may be
necessary.
Controversy, however, still surrounds
treatment options for the asymptomatic patient who has signs of a spinal
anomaly, particularly a milder anomaly such as a thickened filum or an
asymptomatic lipoma. The risks must be weighed, because lipomas of
the filum (or a thickened filum) have much better surgical outcomes than
more complex ones. Some recommend surgical prophylactic untethering in
asymptomatic group, since surgery does not always provide a reversal of
dysfunction or abnormality in symptomatic patients.
Untethering surgery can arrest the
progression of symptoms in the majority of patients; a smaller
percentage of patients show improvement after untethering. Improvement
is more likely to be seen in patients whose primary symptom is pain,
although these patients tend to be an older population including young
adults and adults.
Finding of a normal bony lamina may
allow identification of the dura and subsequent improved understanding of
abnormal tissue planes. This principle holds true intradurally as well,
where rostral exposure of normal spinal cord may facilitate safer
dissection. Use of laser, if available, helps in complex cases, such as,
a lipoma.
Intra-operative neurophysiologic
monitoring with combinations of motor-evoked potentials and
sensory-evoked potentials helps in identifying nerve roots.
The filum is recognizable by its fatty
appearance, by its straight midline location, and by its vasculature. It
is important to visualize the underside of the filum before sectioning,
because nerve roots can travel along with the filum. Once the filum is
sectioned, care should be taken that there is no bleeding at the site of
section before the proximal stump is released, because it may retract
out of reach.
In split cord malformations, once the
median spur/septum is removed, the dural sleeves of both hemicords are
opened, and the median dura is resected along with the ventral dura with
no ventral repair.
Dermal sinus tracts should be
explored intradurally and excised in toto. Simple excision of the
extradural component of the tract may not alleviate intradural tethering.
As much of any intradural lipoma or
dermoid is excised as is safely possible. It is not desirable to embark
upon a hazardous total excision especially in the caudal and transitional
variant of lipomyelorneningocoele. The lipomas dissected all around under
the operating microscope, defining its neck in the lumbo-dorsal fascia.
The normal dura under the laminectomy is opened and extended inferiorly
dividing the constricting fibro vascular band lying around the dura just
inferior to the last intact lamina. The band is frequently seen in the
dorsal variant and its division leads to an immediate ballooning of the
released dura. An associated congenital dermal sinus or split cord
malformation is treated. Likewise any intraspinal meningocoele may have
to be excised before opening the dura. On opening the dura, extreme care
is required to preserve sensory nerve roots that may be adherent to the
dura. Careful dissection using magnification and bipolar stimulation is
required to identify and excise the filum terminale to release the
tethered cord. As this is being done one may notice an actual cephalic
migration of the cord and a change in the direction of the nerve roots.
Neurenteric cysts often are extremely
adherent to the spinal cord and, because of the risk of recurrence,
should be resected completely if possible. This may require a ventral
approach.
A watertight dural closure, with graft
if necessary, is mandatory. To avoid retethering, meticulous attention is
needed in hemostasis and closure. Dural closure with 4-0 Nurolon is
adequate for many straightforward detethering operations. With more
complex spinal dysraphisms, a running monofilament suture can be used
with good results. In complex lesions, such as extensive lipomas,
resection of the maximal amount of pathologic tissue should be performed,
followed by imbrications of the pial surface to create a smooth surface
apposed to the dura.
In severe cases of retethering in
patients who have myelomeningocele and compromised neural function,
transaction of the spinal cord above the neural placode can be performed
to prevent the placode from scarring and forming adhesions. Post
operatively, patients are nursed prone to minimize adhesions of the cord
to the dural suture line. The patients are turned supine and slowly
elevated in bed over several days.
Retethering is about 20% in complex
cases. Complete hemostasis at surgery, imbrications of the pial
surface to create a smooth surface apposed to the dura, and nursing
patients in prone position postoperatively help in preventing
retethering. Any new or significantly progressive orthopedic, urologic,
or neurologic symptom should be evaluated for the possibility of
retethering. Although retethering can occur at any time, the risk often
decreases once adult stature is reached and growth has stopped. Resurgery
may be considered.
|