|
These rare but interesting lesions
comprise 1 per cent of all brain 'tumors'. The spectrum of developmental
cysts in CNS include, arachnoid cysts, ependymal cysts, colloid cysts,
dermoid and epidermoid cysts, Dandy walker cysts ,epithelial cysts, and
porencephalic cysts. They are mostly intracranial, but can occur
intraspinally as well.
1) ARACHNOID CYSTS
(leptomeningeal cysts):
They were referred by Richard Bright in
1831 as ‘serous cysts of the arachnoid’; he described cystic lesions with
liquid, clear, contents and intra-arachnoidal localization. About
127 years later, Starkman described the intra-arachnoid localization of
these cysts, giving an explanation for their etiology.
Epidemiology:
Arachnoid cysts correspond to about 1%
of all intracranial lesions in the general population and about 3% in the
pediatric population. Although 60-90% of reported cases have been
found in young people (less than 20 yeas old), it is possible to find
them in the elderly.
The sex distribution shows a preference
for the males, in the ratio of 3:1 and for left side of the CNS
structures.
Etiology:
They are congenital lesions that
probably arise during development from splitting or duplication of this
membrane.
The etiology of these lesions is
extremely controversial.
Intra-arachnoid cyst theory theory
suggests a congenital origin for the etiology of the arachnoid cysts; at
some time during development, there is a duplication of the arachnoid
membrane, proliferation of the arachnoid cells, forming a cavity which
later fills with CSF through several mechanisms. This was defended
by Starkman and suggested by Bright in 1831. Frequently these cysts are
associated with venous anomalies and the developmental anomaly probably
occurs between the sixth and eighth week of fetal life, precisely when
the vascular structures begin their development.
Shreiber, suggested that most arachnoid
cysts were caused by incomplete canalization of the subarachnoid space
with the formation or pouch like spaces which become distended with CSF
consequent upon arterial pulsation.
Dott and Gillingham, also postulated
that such pouches usually form along the axis of a main cerebral artery,
that the leptomeningeal space at the distal boundary of a cistern becomes
occluded and that the fluid propelled into the proximal portion cannot
escape over the convexity of the brain.
The striking and nearly invariable
association of arachnoid cysts with normal subarachnoid cisterns has led
to hypothesize that arachnoid cysts represent a congenital anomaly
of the developing subarachnoid cisterns in early intrauterine life. It is
postulated that, during the process of the complex folding of the
primitive neural tube and the formation of normal subarachnoid cisterns,
an anomalous splitting of the arachnoid membrane occurs.
Cysts of the sellar region (supra, para
and intrasellar) are considered to be an extension of the Lillequist
membrane, which divides the chiasmatic cistern of the interpeduncular
cistern, being associated with alterations of the normal CSF flow.
Cysts of the posterior fossa are
probably caused by various degrees of obstruction of the foramens of
Luschka or Magendie. Intraventricular cysts are explained by
mesenchymatous invagination through the choroidal tissue that is formed
by digitations of pia-mater with arachnoidal stroma.
Spinal intradural cysts are related to
the septum posticum at a thoracic level, and thoracolumbar extradural
cysts are explained as invaginations of the arachnoid through small
pre-existent dural defects.
The fact that the arachnoid cysts occur
mainly in young people (less than 20 years old) with a strong incidence
in the first years of life as well as the occurrence of case reports with
a familiar incidence and the frequent association of these cysts with
diseases related to chromosome alterations (polycystic kidney,
neurofibromatosis, chromosome 12 trisomy) reinforce the possibility of a
congenital origin for these lesions.
Apart from the structural alterations
there is an alteration of the normal CSF flow, as confirmed in some
cases, by the persistence of symptoms in patients whose cysts were
surgically removed.
Pathology:
|
An arachnoid cyst is by
definition, a benign lesion, with well defined outlines, within the
arachnoid membrane or covered by layers of arachnoid cells supported by
collagen fibres, having liquid contents similar to CSF. It is a
cavity, whose walls are formed by arachnoid cells (simple or multiple
layers), supported by a stroma, rich in collagen fibres.
They can develop anywhere along
the cerebro-spinal axis, but almost all occur in relation to an
arachnoid cistern.
The distribution of arachnoid
cysts in two hundred and eight reported cases is as follows:
Sylvian fissure, 49%;
cerebellopontine angle, 11%; supracollicular area, 10%; the vermis, 9%;
sellar and suprasellar area, 9%; interhemispheric fissure, 5%; cerebral
convexity, 4%; and the clival and interpeduncular area, 3%.
|
|
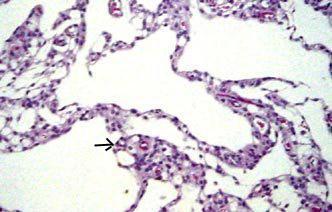
|
|
Lobulated
arch.cyst(H&E)- lining of meningothelial (arrow)
cells supported
by collagen fibres.
|
|
A lesser percentage occurs within the ventricular
system. Intrasellar cysts are the only intracranial arachnoid cysts that
are extradural. Intraspinal cysts are rarer.
The structural features of the arachnoid
cyst wall that distinguish it from the normal arachnoid membrane are
(1) splitting of the arachnoid membrane at the margin of the cyst,
(2) a very thick layer of collagen in the cyst wall,
(3) the absence of traversing trabecular processes within the cyst, and
(4) the presence of hyperplastic arachnoid cells in the cyst wall, which
presumably participate in collagen synthesis.
Most of the small arachnoid cysts do not
increase in size and can be asymptomatic, but the larger cysts can become
bigger and produce symptoms.
The possible reasons for the increase in
size:
The active secretion through the cyst
wall inside the cavity
Unidirectional valve mechanism (ball
valve) with CSF flow from the sub-arachnoid space into the cyst
The active osmosis through the wall by a
similar mechanism to that which occurs at the Pacchionian granulations
Pulsations of the CSF and of the
intracranial arteries transmitted to the cyst cavity through a wide
opening into the sub-arachnoid space.
Transudation through the wall cyst from
the choroids plexus (in the intraventricular cysts).
It is not known which mechanism is
responsible for the increase of the cyst volume, which is probably
multifactorial. However in intraventricular cysts related to the
choroid plexus, the principal mechanism is the transudation through the
cyst wall from plexus secretion. For cysts that are in
communication with the sub-arachnoid space, the principal mechanism is
likely to be the ‘bell valve’ effect and in non-communicating cysts,
active secretion mechanism or osmosis may be more likely.
A classification based on cisternography
with metrizamide and CT isotopic cisternography and, more recently cine
MRI, defines the existence of communication with the subarachnoid space
and the cavity of the cyst. The cyst can then be as slowly or rapidly
communicating or non communicating (real cysts).
A classification based on etiology
divides the cysts into primary (congenital) or secondary (traumatic or
infectious origin).
Finally a classification which is based
on the morphology, volume and effect on the CNS structures and bone
structure as suggested by Galassi divides the cysts into 3
types. Type 1 are small asymptomatic cysts, type II have some mass
effect, with bone erosion and type III have deformation of large areas of
the CNS and gross bony changes.
Clinical features:
In many instances, an arachnoid cyst is
an incidental finding. The clinical presentation will depend on the
location and size of the
achnoid cyst, and the symptoms often are
mild considering the large size of some cysts. Most patients will come to
medical attention in the first two decades of life, often in the first 6
months.
These lesions cause symptoms and signs
of increased ICP by compressing the normal tissue and obstructing the CSF
pathway.
The symptoms depend on the cyst
localization. Symptoms and signs include cranial enlargement, localized
cranial bulging, especially the large cysts, can present acutely with
sudden deterioration.
Suprasellar cysts may also present with
endocrine symptoms, head bobbing, and visual disturbances. Either
communicating or obstructive hydrocephalus is often present. In the
elderly, dementia has been described.
Intraspinal cysts may produce a tetra or
para paresis, with abnormal reflexes, sphincter dysfunction, sensibility
alterations and radicular pain according to the level of the lesion.
Investigations:
Arachnoid cysts are usually diagnosed by
CT or MRI. Further evaluation with CSF contrast flow studies is only
occasionally necessary for the diagnosis of midline suprasellar and
posterior fossa lesions.
CT shows a smoothly bordered
noncalcified extraparenchymal cystic mass with density similar to CSF and
no contrast enhancement and also the bony remodeling.
MRI may show the arachnoid membrane and
also differentiates the CSF in the arachnoid cysts from the neoplastic
cysts and ependymal ( usually, intraparenchymal) cysts. Porencephalic
cyst usually communicate into the ventricle. In addition associated
cerebral and cerebellar hypoplasias are well studied.
Deep invagination of an arachnoid cyst
into the cerebral hemisphere may simulate porencephaly to such an extent
that it has been termed pseudoporencephaly. However, the inferior aspect
of the arachnoid cyst shows a displaced but otherwise normal cerebral
cortex, while in porencephaly, the surrounding cortex and white matter
are abnormal.
Management:
The cysts, that do not cause mass effect
and have not changed in size need not be treated.
Many procedures for treatment of
arachnoid cysts have been proposed. Surgical options for arachnoid cysts
include drainage by needle aspiration, craniotomv with excision of the
wall and fenestration into the basal cisterns (open or endoscopically)
with or without a silastic shunt, and shunting of the cyst into the peritoneum
or vascular system.
The Fenestration and shunting are the
two most common options.
Shunting of the cyst material into the
peritoneum or into the vascular system is associated with low morbidity
and mortality and a relatively low rate of recurrence, but the patient
becomes shunt dependant. Long-term complications of a shunt occur in
more than one third of cases. Low pressure shunt is preferred.
Craniotomy with excision of the cyst
wall and fenestration into the basal cisterns permits direct inspection
of the cyst and avoids placement of a permanent shunt in some cases.
However, it is associated with reaccumulation of CSF at the cyst site. In
addition, significant morbidity and mortality may accompany; abrupt
displacement of brain structures following the rapid decompression that
accounts for the unexpected rapid deterioration.
Endoscopic approaches have been used
with good results, although the follow-up period has not been long. The
goal of fenestration is free communication with the basal cisterns.
Shunting the associated hydrocephalus
alone will only worsen the symptoms, and may increase the size of the
arachnoid cyst.
Outcome:
Arachnoid cysts are benign lesions, with
a poorly defined natural history, sometimes with spontaneous disappearance.
Regression of cyst volume post-surgery,
occurs independently of the surgical technique. Sometimes the cyst
volume remains constant, leaving the patient without symptoms.
Usually there is a good clinical and imaging correlation of regression.
For the suprasellar cysts with
hydrocephalus, it is common to have persistent ventricular dilatation in
spite of the cyst having decreased in its volume, with normal
intracranial pressure. This has no consequences in the intellectual
development of the patients. For the sellar cysts it is also common to
have persistence of endocrinological alterations, even with cyst
disappearance.
Arachnoid Cysts by location:
Sylvian fissure/Middle cranial
fossa cysts:
The sylvian fissure is the most common
site for arachnoid cysts (50% of adult cases and 30% of pediatric cases).
They are usually small or medium, but they can become quite large and
open up the fissure to expose the insula and middle cerebral branches.
They usually manifest clinically in children or adolescents, but can
present at any age. Males predominate and the left hemisphere is more
commonly involved.
Global headache, seldom severe, is
common. Macrocephaly or an asymmetric macrocrania is often the
presentation in the young.
Children may present with developmental delay. Acute symptoms of
increased ICP, hemiparesis, or seizures are common.
On CT and MRI, the large cysts extend
posteriorly and open up the sylvian fissure. The temporal lobe may be
underdeveloped. The greater wing of the sphenoid may be displaced
anteriorly and the lesser wing may be elevated. In most cases, the
associated hydrocephalus is due to compression of the 3rd ventricle.
Treatment is either shunting the cyst or
fenestration of the cyst into a cistern or both combined. Symptoms
typically improve after successful treatment. Results are poor when
behavioral abnormalities and mental retardation are present.
Suprasellar cysts:
Suprasellar location accounts for
approximately 10% of arachnoid cysts and less than 1% of intracranial
mass lesions. Cysts in the suprasellar region appear to arise from the
suprasellar cistern. It has been suggested that the cysts may arise from
an imperforate membrane of Lillequist.
Typically, most patients are adolescents,
and present with hydrocephalus. Adults present with visual problems.
Hypopituitarism, especially the growth hormone deficiency, can occur.
Rarely, there may be ‘bobble head-doll’
syndrome. They experience an involuntary two or three times per second
vertical bobbing of the head, with compensatory horizontal movements of
the trunk, attributed to the abnormal pressure exerted by the cyst on the
3rd ventricle and on the dorsomedial nucleus of the thalamus.
On CT and MRI images, a suprasellar arachnoid
cyst appears as a smooth, oval or round lesion in the region of the third
ventricle .
Multiple approaches to suprasellar cyst
fenestration have been attempted, including transfrontal removal of the
anterior portion of the cyst, a transcallosal approach to communicate the
cavity of the cyst into the ventricular system, and insertion of
catheters between the cyst and ventricle or chiasmatic cisterns. Any one
of these operations has a poor chance of prolonged success and may fail
to decrease either the size of the cyst or the ventricular system.
Shunting the associated hydrocephalus
will only worsen the symptoms, and may increase the size of the arachnoid
cyst. A combination of cyst fenestration and ventricular shunting may be
more successful.
Convexity and Interhemispheric
fissure cysts:
Convexity lesions differ from arachnoid
cysts in other locations due to their lack of contact with cisternal
spaces. Determining the origin of interhmispheric cysts can be
difficult. Imaging of the corpus callosum, third ventricle, and
collicular plate is essential, because the third ventricle can herniate
into the interhemispheric fissure, mimicking an arachnoid cyst.
Usually, they are incidental findings.
Adults can present with raised ICP, epilepsy, and focal neurological
deficit. Children may present with localized macrocrania.
CTand MRI demonstrate rounded lesions
with CSF density, overlying the cerebral cortex or interhemisheric area.
Typically, they are treated with
cysto-peritoneal shunting, often with excision of the outer cyst wall.
Most of these cysts have been detected
in patients aged less than 15 years. Symptoms frequently arise from
hydrocephalus secondary to compression of the posterior third ventricle
or the aqueduct.
Quadrigeminal plate arachnoid
cysts:
Pupillary reactivity or eye movements
are disturbed due to compression of the quadrigeminal plate or stretching
of the 4th nerve.
|
CT and MRI show an ovoid
collection posterior to the 3rd ventricle.
Definitive surgical treatment
can be difficult because the region is not easily accessible.
Fenestration followed by shunting may have a better success. Recurrence
is high.
Intraventricular arachnoid
cysts:
The differential diagnosis
includes ependymal cyst, epidermoid cyst, dermoid cyst, infectious
cyst, and porencephalic cyst.
MRI is the definitive study.
Asymptomatic cavum septi pellucidi, and septum cavum veli
interpositi may be considered
normal variants.
|
|
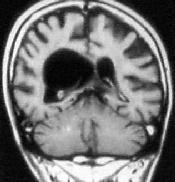
|
|
Intraventricular
arach.cyst
|
|
Simple drainage is usually followed by
reaccumulation. Endoscopic fenestration, craniotomy and cyst excision,
cyst-peritoneal shunting are the surgical options.
Posterior fossa archnoid cysts:
They are less common, about 25% of all
intracranial cysts and usually occur in the midline, superficial to the
vermis or in the CP angle. Less frequent locations include the cerebellar
convexities, or pre pontine areas. The posterior fossa is a common site
of benign intracranial cysts, especially in children.
The differential diagnosis of a
posterior fossa include, arachnoid cysts, Dandy-Walker
malformation, and mega cisterna magna.
|
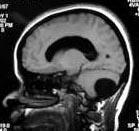
|
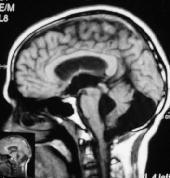
|
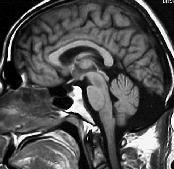
|
|
Post.
fossa arch. cyst- MRI
|
Dandy-walker
cyst-MRI
|
Mega
cisterna magna-MRI
|
|
An
arachnoid cyst results in anterior displacement of the fourth
ventricle, but normal cerebellar development.
|
Dandy-Walker
malformation is a cystic dilatation of the fourth ventricle or a cyst
in communication with 4th ventricle.
|
Mega
cisterna magna is an anatomic variant with normal fourth
ventricle and small cerebellum.
|
Most pediatric patients present with
macro crania. In adults, there are intermittent symptoms or influenced by
posture. CP angle lesions can cause tinnitus or hearing loss.
Fenestration, cyst shunting, and
combination of shunting and fenestration are the treatment options. A
ventricular shunting is often necessary, as the hydrocephalus often fails
to resolve with cyst decompression. Deeper cysts are often
multiloculated, and probably would not respond to shunting and are
usually treated with fenestration.
Spinal arachnoid cysts:
|
Arachnoid cysts also occur
within the spinal canal, in which arachnoid cysts or arachnoid
diverticula may be located subdurally or in the epidural space,
respectively. They are rarer than intracranial cysts. Extramedullary
location is common. They are mostly congenital in origin.
Typically,
spinal arachnoid cysts occur at the midthoracic level and, less
frequently, at the lumbosacral or sacral level. Commonly located dorsal
to the cord. A cyst in this location is usually secondary to a
congenital or acquired defect and is situated in an extradural
location. Intradural spinal arachnoid cysts are secondary to a
congenital deficiency within the arachnoid or are the result of
adhesions resulting from previous infection or trauma.
|
|
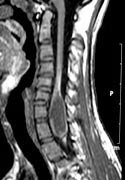
|
|
Intramedullary Spinal
Archnoid cyst
|
|
They cause symptoms indistinguishable
from cord compression due to other causes. Patients with spinal arachnoid
cysts may become symptomatic as a result of local cord displacement or
cord compression. Epidural arachnoid cysts often are associated with
kyphoscoliosis in juveniles. Arachnoid cysts also are associated with
myelodysplasia in spinal dysraphic lesions. Pain produced by intraspinal
arachnoid cysts typically is aggravated by the Valsalva maneuver, which
increases pressure within the cyst.
Remission of symptoms is not uncommon.
Surgical excision is curative.
Tarlov cysts (Spinal perineurial
cyst):
Many consider this a degenerative cyst
and not developmental.
Tarlov described this cyst in 1938,
while conducting an anatomic study of the filum terminale. It is a cystic
dilatation of the subarachnoid space around a nerve root (between
the perineurium of the nerve root and the outer surface of its pia). The
cyst can dissect into the nerve and can contain nerve fibers within
it. These cysts typically involve the sacral nerve roots. It is
found on as many as 5% of lumbosacral MRI.
It is usually
diagnosed incidentally. Approximately, 4 out of 5, are asymptomatic.
Some may present with radicular pain, frequently
occurring in attacks with pain free intervals. Many patients
get relief while by assuming Trendelenberg position in which the patient
is on an elevated and inclined plane, usually about 45°, with the head
down and legs and feet over the edge of the
table. Incontinence.& pain on moving the sacrum are seen in some
patients.
Although a spinal perineurial cyst
involves a single nerve root at first, it may enlarge to the point where
it compress adjacent nerve roots as well.
It is difficult to prove that Tarlov
cysts cause symptoms in many cases because other findings that can cause
these symptoms (disc herniation, stenosis.) are usually found along
with the cyst on MR images or at surgery.
Surgery indicated only if they cause
progressive or disabling symptoms.
Percutaneous CT-guided drainage provides
good relief for several months in most patients but cysts recurs in
almost all. Although the cysts re-pressurize and the patients'
symptoms returned in most cases, this technique seems to be a quick and
simple way of at least attaining a pain-free interval and possibly a
complete cure in some patients.
Sacral laminectomy with microsurgical
cyst fenestration and closure with reinforced epidural fat or muscle
grafts and fibrin glue application is widely employed; lumbar drains for
cerebrospinal fluid diversion for several days postoperatively has also
been suggested.
Secondary (False) arachnoid
cysts:
Arachnoid cysts that are not congenital have
been termed secondary or false cysts. I feel a brief mention of these
acquired cysts is appropriate in this section of developmental cysts.
Such cysts represent accumulations of
CSF resulting from postinflammatory loculation of the subarachnoid space in
patients with head injury, infection, or ICH. The cyst membrane may also
be composed of arachnoid, but in addition, inflammatory cells and
hemosiderin deposits may be present and it is difficult to fenestrate
these cysts.
Lepto meningeal cysts associated with
growing fractures in children are rare. The physiologic brain growth and
CSF pulsations contribute to cyst herniations through a dural rent.
Treatment includes primary repair of the dural rent and the bony defect.
2) EPENDYMAL
CYSTS:
|
These rare cysts arise due to
the inclusion of ependymal cells in the substance of the
brain. They may also originate from ectopic glial tissue present
in the subarachnoid space. Ependymal cysts are common in
oral-facial-digital syndromes.
These later take up a secretory
function resulting in the formation of a cyst. These are
intracerebral and usually adjacent to the ventricles with which they
may occasionally communicate.
The gross appearance is usually
indistinguishable from an arachnoid cyst. Microscopically, the cyst may
be lined by cells resembling ependyma in some places. They may bear
cilia. Ultrastructural studies confirm the neuroepithelial origin of
these cysts.
|
|
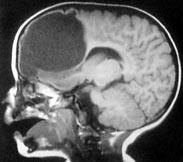
|
|
Frontal
ependymal cyst
|
|
Colloid cysts of the third ventricle have also been
termed neuroepithelial cysts. Cysts in the fourth ventricle which
were lined by cells similar to ependymal cells and cuboidal cells
resembling the epithelium of the choroid plexus have been reported.
Similar choroid plexus cysts may also occur in the third ventricle and
block the foramen of Munro.
Excision is required in only the
symptomatic cases. The rest may be followed up periodically.
3)
RATHKE'S CLEFT CYSTS:
These cysts were first described by
Martin Heinrich Rathke (1793–1860), a German anatomist. Rathke's cleft
cysts are benign, nonneoplastic lesions that are believed to be remnants
of Rathke's pouch, which is the superiorly directed evagination from the
stomadium of the 4 week old human embryo; all but the cranial portion of
the pouch becomes obliterated by week 7 of gestation. The anterior wall
of the remaining cavity becomes the anterior pituitary, while the
posterior wall becomes the pars intermedia of the gland.
Rathke’s cleft cysts are primarily
intrasellar and are found in 13% to 23% of postmortem examinations.
Rathke's cleft cysts are uniloculate and
thin-walled and contain watery to mucinous fluid. Light microscopy of the
lining shows goblet, ciliated and, to a lesser extent, secretory cells of
anterior pituitary type. The finding of ciliated epithelial and mucous
secreting cells in a pituitary gland are pathgnomonic for Rathke's cleft
cysts.
Radiologically, they may mimic
craniopharyngiomas or as cystic pituitary adenoma.
Plain skull radiographs do not, usually,
reveal a enlarged sella turcica. In patients with symptomatic Rathke's
cleft cysts , plain skull radiographs commonly demonstrate findings of an
abnormally configured sella, which varies from slight asymmetry of the sellar
floor to massive erosion. In some patients, intrasellar and/or
suprasellar calcification is observed.
CT reveals an hypodense cystic mass
lesions arising from the pituitary fossa, without enhancement.
MRI shows cystic lesions with long T1
and long T2 although sometimes intrinsic paramagnetic substances may
produce T1 and T2 shortening resulting in hyperintense T1 and hypointense
T2 lesions. Rathke's cleft cysts usually have a thin wall that may
enhance with gadolinium-based contrast material. Variability in the
gadolinium enhancement among individual cysts may reflect squamous
metaplasia in the wall or a peripherally displaced rim of pituitary
tissue.
Rathke's cleft cysts almost always
are homogeneous in signal intensity, whereas other lesions, such as
cystic craniopharyngiomas and hemorrhagic adenomas, more frequently have
heterogeneous signal intensity.
These cysts are rarely symptomatic and
are usually incidental findings on imaging studies. Occasionally, they
cause symptoms by causing pressure on adjacent structures such as the
optic nerves and the pituitary gland causing visual problems, loss of
pituitary function, hypothalamic dysfunction and headache when they grow
in the suprasellar space. A Rathke's cyst may occasionally be associated
with a pituitary adenoma. Partial excision of the cyst wall and drainage
should result in cure: only a small percentage of such cysts recur.
4) PORENCEPHALIC CYSTS:
|
More appropriate term may be
'hole in the brain'
Porencephalic cysts,
characteristically, communicate with the ventricles or subarachnoid
space and are covered on the outside by arachnoid.
These are congenital (primary)
intracranial cysts and may arise as a leptomeningeal cyst.
It is possible that a failure
of development of a part of the cerebral mantle may result in a
cyst.
In addition to their congenital
origin, porencephalic cysts may also arise as a result of trauma
specially during birth or infancy when following loss of cerebral
tissue adjacent to the ventricles a cyst forms and communicates with
the ipsilateral dilated ventricle.
Puncture porencephaly is the
development of a cystic cavitation along the track of a ventricular
needle, manifesting in course of time, following rise in intracranial
pressure due to nonfunctioning of the shunt in cases of
hydrocephalus.
|
|
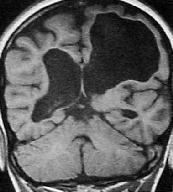
|
|
Porencephalic
cyst
|
|
This may also follow prolonged
ventricular drainage or repeated ventricular punctures.
Surgical excision may be
considered in cases where these cysts are found to be the cause of
intractable epilepsy. The area of excision should include the surrounding
gliosed cerebral tissue as well.
5) Other
developmental cysts are discussed elsewhere.
They are, Colloid cyst of the third
ventricle, Dandy
Walker cyst, and Epidermoids,
Dermoids, & Neuroenteric cysts.
|