|
Almost one fourth of all
deaths attributable to disorders of the nervous system are caused by
hemorrhage in the intracranial cavity. When the bleeding occurs primarily
within the subarchnoid space rather than in
brain parenchyma, the condition is referred to as subarachnoid
hemorrhage. It is more a clinical syndrome than a clear pathological
entity.
The incidence of
subarachnoid hemorrhage varies considerably. The average age
of patients with SAH is substantially lower than for other types of
stroke, peaking in the sixth decade. Gender, race and region have a
marked influence on the incidence of SAH. Women have a 1.6 times higher
risk than men, and black people a 2.1 times higher risk than whites. In
Finland and Japan, the incidence rates are much higher than in other
parts of the world. In Japan the incidence is
25 per 1,00,000 general population and in U.S.A.
it is 16 per 1,00,000. The incidence is about 4 per 1,00,000
and is on the increase in India. Considering
our population figures, one can imagine the total number of patients in
any medical practice.
Risk factors:
Dietary, hereditary,
socio-economic factors may have a role in the pathogenesis of this
disorder.
Only smoking, hypertension and heavy
drinking, and use of oral contraceptives are accepted as significant risk
factors which can be modified. The risks are not clear for hormone
replacement therapy or an increased level of plasma cholesterol.
An important risk factor is familial
predisposition to SAH. Between five and 20% of patients with SAH have a
positive family history. First-degree relatives
of patients with SAH have a 3- to 7-fold increased risk of being struck
by the same disease. In second degree relatives, the incidence of SAH is
similar to that found in the general population.
The occurrence of SAH is also associated
with specific heritable disorders of connective tissue, but these
patients account for only a minority of all patients with SAH. Even though
autosomal dominant polycystic kidney disease (ADPKD) is the most common
heritable disorder associated with SAH, it is found in only 2% of all
patients with SAH. Aortic stenosis and
polycystic kidney are the only 2 congenital anomalies with correlation
with aneurysms. They may have congenital origin, but they also cause high
BP which may be a factor. Other genetically determined disorders
include, EhlersDanlos disease IV, Marfan's syndrome, Lupus erythematosis, and neurofibromatosis type 1;
but these associations are weaker than between ADPKD and aneurysms and
these syndromes are seldom found in patients with SAH.
Clinical features:
Perhaps nowhere else in
medicine, history is so very important.
A sudden, severe headache
that is unlike any the patient has experienced previously, is due to
subarachnoid hemorrhage until proved otherwise. It may be generalized or
localized and associated with nausea or vomiting.
Classically, the headache from
aneurismal rupture develops in seconds. However, it is to be noted that
even an accurate history does not reliably distinguish between aneurismal
rupture and innocuous forms of headache, such as benign vascular headache
or a muscle contraction headache. First, only half the patients with
aneurysm rupture describe the onset as instantaneous, the other half
describe it as coming on in seconds to even a few minutes. Secondly, in
the group of patients whose headache came on within a split second,
innocuous forms of headache outnumber SAH by 10 to one. If explosive
headache is the only symptom, the chance of SAH being the cause is only
10. Also, preceding bouts of similar headaches are recalled in 20% of
patients with aneurismal rupture and 15% of patients with innocuous
thunderclap headache.
Vomiting occurs in 70% of patients with
aneurismal rupture, but also in 43% of patients with innocuous
thunderclap headache.
Kerning's sign may appear
6-24 hours later. It
does not occur if patients are in deep coma. Mild
temperature elevation, photophobia and hypertension are not uncommon.
Dizziness, true vertigo or
fatigue may also occur as may memory impairment, confusion or agitation. 1 to 2% of
patients with SAH present with an acute confusional
state and in most such patients a history of sudden headache is lacking.
Epileptic seizures at the onset of
aneurismal SAH occur in 616% of patients; however, the majority of
patients with de novo epilepsy above age 25 years will have
underlying conditions other than SAH, but the diagnosis should be suspected
if the postictal headache is unusually severe.
Ophthalmologic findings may
be observed in more than one third of patients. Intra ocular hemorrhage
in the subhyloid or preretinal
space are more characteristic of subarachnoid hemorrhage and occurs in 17% of
patients who reach the hospital. III, V &
VI nerve Palsies and other focal neurological deficits may develop,
depending on the area of brain involved and may be secondary to intraparenchymal bleeding, ischemia, thromboembolism,
subdural hematoma or obstruction to CSF pathways.
About one third of patients
will have a minor leak referred to as 'sentinel hemorrhage'. Headache is
the most common symptom. Other warning signs include impairment of ocular
movements, motor or sensory impairment.
Grading of
subarachnoid hemorrhage has centered about complaints of headache and the
patients level of consciousness.
|
Grade
|
Hunt and Hess modification of Botterell's (1968)-widely used
|
WFNS grading,(1988)-under Charles Drake
|
|
1
|
Asymptomatic or a
minimal headache and slight nuchal stiffness.
|
GCS 15, no motor
deficit.
|
|
2
|
Moderate to severe
headache - no neurological deficit.
|
GCS 14-13, no
motor deficit.
|
|
3
|
Drowsiness,
confusion or mild focal deficit.
|
GCS 14-13, motor
deficit.
|
|
4
|
Stupor, moderate
to severe hemi paresis.
|
GCS
12-7 with or without motor deficit.
|
|
5
|
Deep coma, decerebrate rigidity, moribund appearance.
|
GCS 6-3 with or without
motor deficit.
|
Cranial nerve
palsies are not considered a focal deficit.
Serious systemic diseases
such as hypertension, diabetes, chronic pulmonary disease, angiographically evident vasospasm place the patient
in the next less favorable grade.
On occasions the history and clinical
examination may suggest the cause of SAH.
Causes
of SAH:
85% of SAHs are attributable to saccular
aneurysms; 10% are caused by non-aneurysmal SAH and the remaining 5% by a
variety of rare conditions.
Saccular
('Berry') aneurysms (85%):
The saccular 'berry' aneurysms and other
causes of aneurysms are discussed
elsewhere.
Idiopathic non-aneurysmal perimesencephalic
hemorrhage (10%):
This harmless variety is defined only by
the characteristic distribution of the extravasated
blood on brain CT, in combination with the absence of an aneurysm. The extravasated blood is confined to the cisterns around
the midbrain, and the centre of the bleeding is
immediately anterior to the midbrain. In some cases, the only evidence of
blood is found anterior to the pons. There is no extension of the
hemorrhage to the lateral sylvian fissures or
to the anterior part of the interhemispheric fissure. Some sedimentation
of blood in the posterior horns of the lateral ventricles may occur.
There is no frank intraventricular hemorrhage.
Perimesencephalic hemorrhage can
occur in any patient over the age of 20 years, but most patients are in
their sixth decade. A history of hypertension may be obtained. In
one-third of the patients, strenuous activities immediately precede the
onset of symptoms, a proportion similar to that found in aneurismal
hemorrhage.
Clinically, there is little to
distinguish idiopathic perimesencephalic haemorrhage from aneurysmal hemorrhage. The headache
onset is more often gradual (minutes rather than seconds) than with
aneurysmal hemorrhage, but the predictive value of this feature is poor.
Loss of consciousness and focal symptoms are exceptional and then only
transient; a seizure at onset virtually rules out the diagnosis. On
admission, all patients are, in fact, in perfect clinical condition,
apart from their headache. Transient amnesia is found in about one-third
and is associated with enlargement of the temporal horns on the initial
CT scan. Typically, the early course is uneventful: rebleeds
and delayed cerebral ischaemia simply do not
occur. Only few have symptoms from this ventricular dilatation and even
then an excellent outcome can be anticipated. The period of convalescence
is short and almost invariably patients are able to resume their previous
work and other activities. Rebleeds after the
hospital period have not been documented thus far and the quality of life
in the long term is excellent.
A perimesencephalic
pattern of hemorrhage may occasionally (in 2.55% of cases) be caused by
rupture of a posterior fossa aneurysm. The chance of finding an aneurysm
is about 5%. In recent years, CTA has been studied as a method to confirm
or exclude the presence of an aneurysm in patients with a perimesencephalic pattern of hemorrhage on CT.
Rare causes of SAH (5%):
Trauma:
Trauma may confuse the issue, especially
with no external wounds to indicate an accident, with a decreased level
of consciousness or with retrograde amnesia, making it impossible to
obtain a history. CT may reveal associated contusions, fractures, and
unusual locations of subarachnoid blood.
In patients with previous head injury,
and particularly with a skull fracture, a dural
arteriovenous malformation (AVM) should be suspected, since healing of
the fracture may be accompanied by the development of such a
malformation.
Pituitary
apoplexy:
The initial features are a sudden and
severe headache, with or without nausea, vomiting, neck stiffness or a
depressed level of consciousness. A combination of visual and oculomotor
deficits should raise the suspicion of a pituitary apoplexy. Usually, the
underlying adenoma has insidiously manifested itself before the dramatic
occurrence of the hemorrhage by a dull retro-orbital pain, fatigue, a gradual decrease of visual acuity or a constriction
of the temporal fields.
The precipitating event of arterial
hemorrhage occurring in a pituitary tumor is thought to be tissue
necrosis, involving one of the hypophyseal
arteries. Several contributing factors may precipitate hemorrhagic
infarction of a pituitary tumor, such as pregnancy, raised intracranial
pressure, anticoagulant treatment, cerebral angiography or the
administration of gonadotrophin-releasing hormone.
Intracranial
AVMs:
Subarachnoid bleeding at the convexity
of the brain may occur from superficial cerebral AVMs, but only in <5% of all ruptured
AVMs is the extravasation only in the subarachnoid space, without
intracerebral hematoma. Saccular aneurysms form on feeding arteries of
1020% of AVMs, presumably because of the greatly increased flow and the
attendant strain on the arterial wall. If bleeding occurs in these cases,
it is more often from the aneurysm than from the malformation. In those
cases the site of the aneurysms is different from the classical sites of
saccular aneurysms on the circle of Willis and again the hemorrhage is
more often into the brain itself than into the subarachnoid space.
The risk of hemorrhage from dural
AVMs depends on the pattern of venous drainage. Patients with direct
cortical venous drainage have a relatively high risk, which is further
increased if a venous ectasia is present. Patients with drainage into a
main sinus have a low risk of hemorrhage and if no reflux occurs into the
smaller sinuses or cortical veins, it is negligible. After a first
rupture, rebleeding may occur.
Arterial
dissection:
Dissection, in general, tends to be
recognized more often in the carotid than in the vertebral artery, but
SAH from a dissected artery occurs mostly in the vertebral artery.
Neurological deficits that may accompany
SAH from vertebral artery dissection are palsies of the ninth and tenth
cranial nerves, by subadventitial dissection,
or Wallenberg's syndrome. Rebleeds occur in
between 30 and 70% of cases. The interval can be as short as a few hours
or as long as a few weeks. The second episode is fatal in approximately
half of the patients.
Dissection of the intracranial portion
of the internal carotid artery or one of its branches as a cause of SAH
is much less common than with the vertebral artery. Reported cases have
affected the terminal portion of the internal carotid artery, the middle
cerebral artery.
Drug
abuse:
The source of SAH in drug abusers
without an aneurysm is unknown, although vasculitis has been suggested.
In patients with SAH related to the use of cocaine, 70% have an
underlying aneurysm. CT may simulate SAH due to saccular aneurysm.
Coagulopathies:
Anticoagulant drugs are seldom the sole
cause for SAH. Severe coagulopathy other than by anticoagulant drugs,
e.g. congenital deficiency of factor VII, is also a rare cause of SAH. If
aneurysmal hemorrhage occurs in a patient on anticoagulants, the outcome
is relatively poor.
Thirty per cent of patients with sickle
cell disease and SAH are children. CT scans in these children show blood
in the superficial cortical sulci; angiograms show no aneurysm, but often
show multiple distal branch occlusions and a leptomeningeal collateral
circulation. The SAH is attributed to rupture of these collaterals. The
outcome is poor. Most adult patients in whom sickle cell disease
underlies SAH have a ruptured aneurysm at the base of the brain.
Superficial
siderosis of the CNS:
There is no sudden headache. The
clinical syndrome is almost invariably characterized by sensorineural
deafness (95%), furthermore by cerebellar ataxia (88%) and pyramidal
signs (76%). Possible other features include dementia, bladder
disturbance and anosmia. Men are more often affected than women (3:1).
This condition is characterized by iron overload of the pial membranes, through chronic oozing of
blood from any source in the subarachnoid space. Other causes of chronic
bleeding include a CSF cavity lesion or cervical root lesion, a vascular
tumor or any other vascular abnormality.
The high iron content of the pial membranes causes a characteristic signal on MRI
scanning.
Spinal
causes:
10% of the spinal AVMs present
with SAH. In >50% of these patients, the first hemorrhage occurs
before the age of 20 years. There may be a sudden and excruciating pain
in the lower part of the neck, or pain radiating from the neck to the
shoulders or arms. In the absence of such symptoms, the true origin of
the hemorrhage emerges only when spinal cord dysfunction develops, after
a delay that may be as short as a few hours or as long as a few years.
A history of even quite minor neck trauma or of sudden, unusual
head movements before the onset of headache may provide a clue to the
diagnosis of vertebral artery dissection as a cause of SAH.
Rebleeds may occur, even
repeatedly. If a spinal origin of the hemorrhage is suspected, MRI are
the first line of investigation, because spinal angiography is
impractical without localizing signs or symptoms. Saccular aneurysms of spinal
arteries are extremely rare, with recorded incidents in
12 patients. As with AVMs of the spinal cord, the clinical features of
spinal SAH may be accompanied by those of a transverse lesion of the
cord, either partial or complete.
Investigations:
CT scan of
the brain (plain) confirms the bleed and suggests the site and probable
cause of bleed.
It is the procedure of
choice because
of the characteristically hyperdense appearance
of extravasated blood in the basal cisterns.
The pattern of hemorrhage often suggests the location of any underlying
aneurysm, although with variable degrees of certainty. A false-positive
diagnosis of SAH on CT is possible in the presence of generalized brain
edema, with or without brain death, which causes venous congestion in the
subarachnoid space and in this way may mimic SAH. CT studies,
currently, are negative in 2% of patients with SAH.
CT angiography (CTA) is based on the
technique of spiral CT. It can easily be obtained immediately along with
routine non-contrast CT upon which the diagnosis is first made. It is
minimally invasive because it does not require intra-arterial
catheterization. Compared with MRA, it involves radiation and it requires
injection of iodine-based contrast, but is much simpler to perform,
especially in ill patients. In addition, maximum intensity projection
(MIP) images derived from CTA can be rotated and studied on a computer
screen at every conceivable angle, which is a great advantage over the
limited views with conventional angiography.
MRI scan with FLAIR
(fluid attenuated inversion recovery) techniques demonstrates SAH in the
acute phase as reliably as CT, but MRI is impracticable because the
facilities are less readily available than CT scanners, and restless
patients cannot be studied unless anesthesia is given. After a few days
(up to 40), however, MRI is increasingly superior to CT in detecting extravasated blood. This makes MRI a unique method
for identifying the site of the hemorrhage in patients with a negative CT
scan but a positive lumbar puncture, such as those who are not referred
until 1 or 2 weeks after symptom onset.
MR angiography (MRA) is safe, but
less suitable in the acute stage, because in the acute stage patients are
often restless or need extensive monitoring. A recent review of studies
suggest a sensitivity in the range of 69100% for detecting at least one
aneurysm per patient. For the detection of all aneurysms the sensitivity
is 7097%, with specificity in the range 75100%. Despite its
limitations, MRA is a feasible tool for detecting aneurysms in relatives
of patients with SAH.
Transcranial Doppler (TCD) can be combined
with echo imaging (duplex technique) and with colour
coding (transcranial colour-coded duplex
sonography). It helps
in dynamic assessment of the functional status of the circle of Wills and
complement angiography. A recent modification of color Doppler
called Color Doppler Energy or Power Doppler offers greater sensitivity
to flowing blood than standard color flow imaging. The sensitivity of
power Doppler increases further by using an ultrasonic contrast agent,
but even then the sensitivity is only 55% with a corresponding 83%
specificity. Another drawback of this technique is that 15% of patients
have no adequate bone window, which prevents adequate insonation.
Also, the technique is highly dependent on the skills of the operator.
Somato
sensory evoked potential (SSEP) pre operatively,
intra operatively and post operatively gives good indication of brain
ischemia.
Lumbar puncture is an
indispensable step in the exclusion of SAH in patients with a convincing
history and negative brain imaging. At least 6 and preferably 12 hrs should have elapsed between the onset of headache
and the spinal tap, for sufficient lysis and formation of bilirubin and
oxyhemoglobin, the pigments that give the CSF a yellow tinge after
centrifugation (xanthochromia). Xanthochromia is a critical feature in the distinction
from a traumatic tap, and are invariably detectable until at least 2
weeks later. The `three tube test' (a decrease in red cells in
consecutive tubes) is notoriously unreliable, and a false-positive
diagnosis of SAH can be almost as invalidating as a missed one. Spinning
down the blood-stained CSF should be done immediately; otherwise
oxyhemoglobin will form in vitro. If the supernatant appears
crystal-clear, the specimen should be stored in darkness until the
absence of blood pigments is confirmed by. Although the sensitivity and
specificity of spectrophotometry have not yet been confirmed in patients
with suspected SAH and a negative CT scan, it is the best technique
currently available.
There is no scientifically sound method
to distinguish reliably between blood caused by
a traumatic tap from blood that was already present. Even the smoothest
puncture can end in a vein.
Cerebral angiography is still the
gold standard for detecting aneurysms; but this procedure can be time
consuming and it is not an innocuous procedure with a complication rate
(transient or permanent) of 1.8%. At any rate, the aneurysm may
re-rupture during the procedure, as occurs in 12% of cases overall. The
rupture rate in the 6 hrs period following
angiography has been estimated at 5%, which is higher than the expected
rate.
Given the risk of a later rebleed, it is in patients with an aneurysmal pattern
of hemorrhage on CT that repeat angiography seems to be most clearly
indicated. The combined yield of a second angiogram is about 17%. If a
second angiogram again fails to demonstrate the suspected aneurysm,
perhaps a third angiogram may be positive, after an interval of several
months.
There is no doubt that catheter
angiography is on its way out for the pre-treatment assessment of
cerebral aneurysms, as the techniques of CTA and MRA are still improving
and as neurosurgeons and interventional radiologists are growing familiar
with them.
|
|
|
|
|
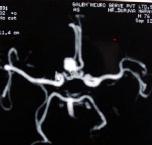
|
|
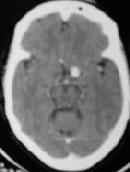
ICA. An bleed -CT
|
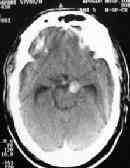
P.C. AN bleed
-CT
|
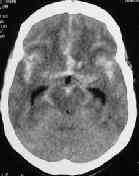
A.com.A.bleed
-CT
|
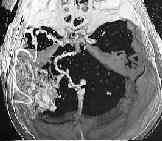
A gaint AVM
- 3D CT
|
A.COM.AN
-3D-CT
|
|
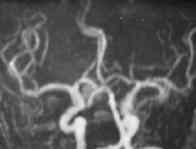
|
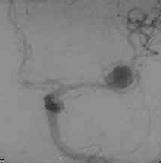
|
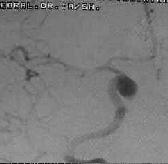
|
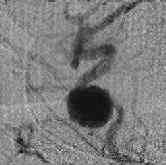
|
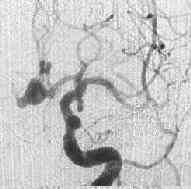
|
|
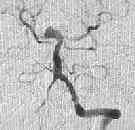
A.COM Art.An
-MRA
|
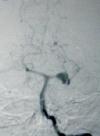
MCA.AN
-angio AP
|
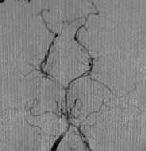
Opth.An
-angio AP
|
Intracav.
AN
-angio lat
|
A.com.An-
nipple sign
-angio AP
|
|
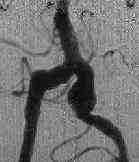
|
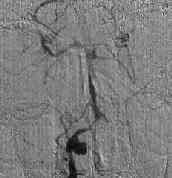
|
|
|
|
|
Vert.Fusiform
An
-angio AP
|
PICA An
-angio AP
|
Basilartip.An
-angioAP
|
P.C.An -angio AP
|
Severe vasospasm
-angio AP
|
Management:
The initial management is
medical.
The aim is to preserve
residual brain function and prevent neurological and systemic
complications.
Bed rest, adequate
analgesics and sedation and careful
attention to fluid and electrolyte balance are mainstay in medical
management. 40% of them may have respiratory abnormalities during initial
bleed, and assisted ventilation for an hour or so helps.
Intracerebral hematomas (ICH) occur in
up to 30% of patients with ruptured aneurysms. Immediate evacuation of
the hematoma should be seriously considered with simultaneous clipping of
the aneurysm if it can be identified, often with the aneurysm having been
demonstrated only by MR angiography or CT angiography. An acute subdural
hematoma, which is usually associated with recurrent aneurysmal rupture
but can also occur with the initial hemorrhage, may be life threatening;
immediate surgical evacuation may be required.
Rebleeding is
one of the most devastating complication of initial hemorrhage with a
maximum incidence between 5th - 9th day. The total risk
of rebleeding without medical or surgical
intervention in the 4 weeks after the first day can be estimated to be
3540%. Between 4 weeks and 6 months after the hemorrhage, the risk of rebleeding gradually decreases from the initial level
of 12% a day to a constant level of 3% a year. Female
gender, advancing age, poor neurological grade, poor medical condition,
moderate to severe (170 - 240 mm Hg) systolic hypertension are some of
the predisposing factors.
In the first few hours after admission
for the initial hemorrhage, up to 15% of patients have a sudden episode
of clinical deterioration that suggests early rebleeding.
At present it is virtually impossible to prevent this from happening, but
surgical or endovascular intervention can prevent recurrent hemorrhages
occurring later.
Use of Antifibrinolytic
agents such as Epsilon Amino Caproic Acid is
not widely accepted these days as studies show no change in final
outcome. Although
the risk of rebleeding was significantly
reduced by antifibrinolytic therapy, but this
was offset by a similar increase of the risk of secondary cerebral
ischemia.
Vasospasm is another dreaded complication with an incidence of
about 35%, and has been blamed for delayed cerebral ischemia. It develops
between 4-14 days with a peak incidence between 6th-8th day and lasts for up to 2 weeks.
Neither the amount of
subarachnoid blood nor the angiographically
demonstrated arterial narrowing can predict the severity. The narrowing in
distal branches can escape a transcranial doppler
study.
It is manifested by
decreased by level of consciousness and fever followed by focal symptoms
and signs. The deficits may remain unchanged, resolve within a few days
or progress to cause permanent disability or death. Satisfactory
treatment is not available.
Blood volume expansion and
arterial hypertension is being recommended by some, not supported by any
valid study. The HHH
therapy involves, attention to patients fluid balance to keep hematocrit
at around 35% and hemoglobin at 10 -12 mg/dl and maintenance of the
systolic blood pressure at 150 -180 mmHg.
Calcium Entry blockers (Nimodipine), it is claimed, is effective in high doses.
Lately, nimodipine 60mg orally every 4th hourly
for 3 weeks is widely recommended. It is uncertain whether nimodipine acts through neuroprotection, through
reducing the frequency of vasospasm, or both. Other calcium antogonists (Nicardipine
and AT877) definitely reduce the frequency of vasospasm, but the effect
on overall outcome remains unproved. Other strategies to combat the
vasospasm, such as, use of calcitonin-gene-related peptide (a potent vasodilatator), and lysis of the intra-cisternal blood clot with intrathecally
administered recombinant tissue plasminogen activator, are still under
trial.
Prophylactic transluminal balloon
angioplasty, and intra-arterial infusion of papaverine,
following super-selective catheterization have been advocated by some.
Acute non
communicating hydrocephalus is of grave prognostic
significance and has an incidence of about 20% and often requires ventriculostomy. Gradual obtundation
within 24 hrs of hemorrhage, sometimes
accompanied by slow pupillary responses to light and downward deviation
of the eyes, is fairly characteristic of acute hydrocephalus. The role of
early drainage is not well established.
Chronic and sub acute hydrocephalus occurs in 15-20% may require
surgical intervention in only 5 to 10 % of patients.
Management of Blood pressure is an issue in
patients with stroke including SAH. Studies suggest that hypertension
after SAH is a compensatory phenomenon, at least to some extent, and that
it should not be interfered with. It is better to reserve antihypertensive
drugs (other than those the patients were on already) for patients with
extreme elevations of blood pressure as well as evidence of rapidly
progressive end organ deterioration, diagnosed from either clinical
signs, such as, left ventricular failure.
Fluid and electrolyte
abnormalities are common and have
been attributed to the hypothalamic disturbance. Most commonly there is
hyponatremia which may be associated with inappropriate hypersecretion
of ADH and in some due to the so called 'Cerebral salt wasting syndrome'
which appears to be the commoner cause. SIHADH requires fluid
restriction and 'Cerebral salt wasting syndrome' requires fluid
replacement. Diabetes insipidus may result from failure of ADH release
and has a grave prognosis. Treatment may require vasopressin.
Neuroprotectors: Recently
nimodipine and vitamin E are widely used as brain protectors.
N'-propylenedinicotinamide (nicaraven),
and Tirilazad are claimed to have some
neuroprotection. Use of aspirin and other antiplatelet agents have not
shown to improve the outcome. Studies continue.
Other complications include
cardiac abnormalities, respiratory problems, gastro intestinal hemorrhage
associated with Cushings
ulcers and they need close monitoring and treatment.
Surgical clipping:
The aim of surgery is to
prevent rebleed and preserve residual brain function.
Ideally it is clipping of the neck of aneurysms.
Many centers in Japan carry
out surgery as an emergency procedure. But most prefer to do it when the
patient's condition is stable. There is a swing towards early
surgery lately. Ideally patients should be in Grade I condition i.e.,
with no headache and stable blood pressure. At times a hematoma may
require evacuation, irrespective of the grade, and obviously clipping is
carried out along with evacuation.
Various studies suggest that there is no
difference in outcome between early (<3 days), and late clipping. The
surgical clipping is avoided between day 7 and 10 after the initial
hemorrhage. This disadvantageous period for performing the operation in
the second week after SAH coincides with the peak time of cerebral ischaemia and of cerebral vasospasm.
Rarely surgeons fail to
define the neck and are forced to wrap the aneurysm to promote
thrombosis. Various materials such as gauge, acrylic are used. Such
measures are becoming more and more uncommon in this microsurgical era.
Giant aneurysm (more than 2
cm) remains a problem. More often than not, clipping is impossible.
Proximal or Hunterian ligation and trapping the aneurysm with proximal
and distal occlusion of the parent vessel are obvious options. The
results depend on adequate collateral circulation. It has been claimed
that a EC - IC bypass to look after the distal
circulation will make these procedures safe. Despite varying success
rates, trapping procedures remain main stay of treatment of giant
aneurysm. Development of interventional neuroradiology hopefully will
solve this problem.
Occasionally we come across
a surfacing AVM which has caused the SAH and they may be excised. The
problem of rebleed is not such an emergency, as
in an aneurysm.
Interventional radiology:
Endovascular procedures are
increasingly employed these days. Comparisons between endovascular
and surgical clipping is still being debated. In a recent small study,
there is no difference in outcome at 3 months between the surgical group
and the endovascular group.
Rerupture of aneurysms
may occur even months after apparently successful coiling and the
long-term rates of rebleeding after
endovascular coiling still need to be established. A recent study showed rebleeding rates of 0.8% in the first year, 0.6% in
the second year and 2.4% in the third year, with no rebleeding
in subsequent years.
Surgical clipping is not always
definitive either; in a retrospective review of post clipping angiograms,
8% of patients showed aneurysms with a residual lumen or aneurysms that
were previously undetected.
SAH due to Unknown causes:
If angiography is negative, it is
essential to take account of the pattern of hemorrhage on the initial CT
scan. If this pattern is perimesencephalic, the
diagnosis of nonaneurysmal hemorrhage is
established and no repeated studies are needed given the absence of rebleeds and the invariably good outcome. Such
patients need no longer be on an intensive or medium care unit and can be
transferred to a regular ward. Patients with a perimesencephalic
hemorrhage can usually be discharged home after a few days and should be
reassured that no complications will ensue and that they can take up
their lives without any restrictions.
Patients with an aneurysmal pattern of
hemorrhage on CT, but a negative angiography, can still develop secondary
ischemia and have a 10% risk of rebleeds. These
patients should therefore remain on the intensive or medium care unit.
The substantial risk of rebleeding in patients
with an aneurysmal pattern of hemorrhage indicates that, at least in some
patients, an aneurysm escapes radiological detection. Apart from
technical reasons, such as insufficient use of oblique projections, this
phenomenon may have several explanations. Narrowing of blood vessels by
vasospasm has been invoked in some cases. Thrombosis of the neck of the
aneurysm or of the entire sac is another possible reason. Obliteration of
the aneurysm by pressure of an adjacent hematoma may also prevent
visualization, particularly with aneurysms of the anterior communicating
artery.
Conclusion:
Only half a century ago the
exact diagnosis in cases of spontaneous subarachnoid hemorrhage was
rarely established during the patient's life time. At present with
persistent efforts the lesions responsible can be identified in the vast
majority of cases. Reports indicated that 15% of the patients with
subarachnoid hemorrhage don't live long enough to reach the hospital and
about 43% of those hospitalized die within one
month after the event. 42% of such cases are due to rebleed.
Of patients who survive the hemorrhage, approximately one-third remain
dependent. Recovery to an independent state does not necessarily mean
that outcome is good. Various studies suggest that
all in all, only a small minority of all patients with SAH have a
truly good outcome. The relatively young age at which SAH occurs and the
poor outcome together explain why the loss of years of potential life
before age 65 years from SAH is comparable to that of ischemic stroke.
No gains are to be made,
however, by a passive approach in managing the patient with recent
subarachnoid hemorrhage. The severity of this illness and its tendency to
recur and produce death and disability justify an aggressive attempt by
the neurosurgeon to establish a prompt and accurate diagnosis and
treatment.
|