|
Available data regarding epidemiology are limited and they
are less common than the intracranial tumors (about 1:10). In most
series, the average age at diagnosis is 40 years, ranging between 11 days
& 74 years. Both sexes are equally involved. More than 70% of the
tumors were located in the thoracic part or cervical (in that order)
of the spinal cord.
It is convenient to classify the spinal cord tumors by their
location within the spinal cord,
as extramedullary intradural, intramedullary,
and extradural.
Although the extradural tumors do not come under spinal cord
tumors, it is discussed here briefly.
a) Extramedullary intradural tumors
are the commonest spinal cord tumor (84% of all intradural tumors).
Neurofibromas(29%) and meningiomas(25%) are the common ones. Meningiomas are
more common in middle aged women and in the thoracic region. Exophytic
ependymomas and astrocytomas account for about 20%. Sarcomas, vascular
tumors, epidermoids, lipomas etc are occasionally encountered.
b) Intramedullary tumors
are the commonest spinal cord tumors in children. Gliomas (mainly
ependymomas & astrocytomass) make up almost 70% of all intramedullary
tumors. In children astrocytomas are most frequently encountered, in some
material representing up to 81% of such tumors, while in adults alone
ependymomas may account for up to 56% of all intramedullary tumors.
Vascular tumors, represented by hemaingioblastomas and cavernomas, add
upto almost 15% of all intramedullary tumors. Among the other tumors are
found cases of subependymomas, sarcoidosis, neurofibromas, ganglioglioma,
gangliocytoma, oligodendroma and astrogliosis. In the literature there
have also been case reports on primary malignant lymphomas and
neurocytomas in the spinal cord.
Occasionally, malignant tumors from the brain, such as
medulloblastoms, may seed.
|
c) Extradural
tumors are mostly metastatic. They spread into spinal cord from
contiguous structures. About 5% of all patients with cancer develop
vertebral metastasis. Lately, primary non osseous lymphomas are
being reported increasingly.
Bony
tumors can
be divided into two groups: primary, i.e. arising within the bone, and
secondary, i.e. metastatic
to bone. Within the group of primary bone tumors are both benign
and malignant forms.
Benign
tumors are predominantly aneurismal bone cysts and osteoblstomas, whereas
malignant forms include Ewing’s sarcoma, chordomas, chondrosarcomas,
and mesenchymal chondrosarcomas.
Pathophysiology:
|
|
|
|
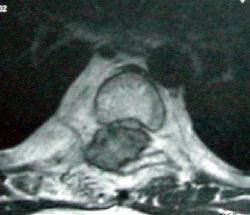
D7 osteoblastoma
|
|
The
tumors can cause symptoms due to compression of the cord and interrupting
the cord's blood supply. Initially, the veins get compressed, resulting
in congestion and edema. Arterial compression occurs later, which may
sometimes lead to distant effects; pressure at the D4 and D5 levels may
cause a greater deficit because of the watershed area in the vascular
supply of the cord at this level.
Direct
pressure on the cord and roots leads to disturbed cord function, the long
tracts being affected early. Lumbar puncture may sometimes cause a shift
in the position of the tumor leading to a sudden increase in the
neurological deficit.. In long standing tumors, there may be gliosis, and
the recovery following surgery may not be satisfactory.
Spinal
meningiomas and other tumors do not, in general, differ from their from
their intracranial counterparts.
However,
gliomas, although share several characteristics with intracranial
gliomas, there are some interesting differences.
Astrocytomas of the spinal
cord are rare neoplasms, about 10 times less common than astrocytomas of
the brain. The average age at diagnosis is between 35 and 40 years.
Astrocytomas of the spinal cord do not show the correlation between
increasing grade and increasing age at diagnosis that is so prominent
with cerebral diffuse astrocytomas.
Spinal
cord astrocytomas are graded according to the same WHO criteria, used for
cerebral astrocytomas, and grade is a strong prognostic indicator.
Low-grade astrocytomas (WHO grade II/IV) comprise about 75 - 90% of
tumors, with the remainder being high-grade astrocytomas (WHO grades
III/IV and IV/IV). Tumors typically involve a focal segment of the cord,
and have a fairly even incidence along its length, but rarely may involve
a large portion of the cord in a condition called "holocord"
astrocytoma. The tumor may grow in a diffuse manner with indistinct
margins between tumor and the adjacent normal spinal cord tissue, and can
extend along spinal nerve roots. Pilocytic astrocytomas have discreet
margins.
An
important feature is the presence of a tumor associated syrinx, which
occurs in about 40% of patients with astrocytomas of the spinal cord.
Syringes are more common with low-grade than high-grade astrocytomas, are
more frequent the further rostral the tumor lies along the cord, and they
appear to favor the rostral aspect of cord above the tumor. Syrinx may be
less common with astrocytomas than with ependymomas. With respect to
syrinx formation, normal CSF flow in the central canal of the cord is
disrupted by the presence of the mass lesion. This mechanical explanation
probably accounts for the fact that tumor-associated syringes are
typically rostral to the tumor.
Ependymomas of the spinal
cord are slightly more common than spinal cord astrocytomas. The average
age of patients is between 35 and 45 years, an age which is higher than
for intracranial ependymomas.
Spinal
ependymomas are thought to arise from ependymal cells lining the central
canal. Cellular ependymomas are distributed evenly along the length of
the spinal cord, whereas myxopapillary ependymomas occur almost
exclusively at the filum terminale and occasionally the conus medullaris.
Tumors may extend over several spinal segments, and may have a
substantial exophytic component. Holocord lesions are rare. Syringes or
tumor-related cysts may be more common with ependymomas than with
astrocytomas of the spinal cord. The lesions are usually well
circumscribed.
Histopathological
classification includes myxopapillary ependymoma (WHO grade I/IV), ependymoma
(WHO grade II/IV) and anaplastic ependymoma (WHO grade III/IV). The two
low-grade lesions are more common than anaplastic ependymoma. Anaplastic
ependymomas may be associated with leptomeningeal spread, although this
complication occurs with the lower grade lesions as well. Ependymomas,
including those arising from the spinal cord, have the unusual propensity
to spread outside of the neuraxis. This is particularly true for
subcutaneous myxopapillary tumors that arise over the sacrococcygeal
region. Metastasis to lung, skin and kidney have been documented.
Low-grade
ependymomas of the spinal cord are usually slowly growing lesions with
little tendency to undergo anaplastic progression to higher grades of
histology or more aggressive biological behavior.
Clinical features:
Spinal cord tumors produce symptoms due to compression of
nerve root or cord, and ischemia vascular compression.
Tethering of the cord by the dentate ligaments and filum
terminale may result when expanding lesions oppose this resistance.
The
main symptoms are, pain, weakness, sensory disturbance, and autonomic
disturbances. In addition, there may be a vertebral deformity, especially
in children.
Extradural tumors mimic
the commoner extramedullary tumors; the root pain is well defined. The
pain is aggravated by coughing and sneezing and other spinal movements.
Autonomic disturbance is rare, unless it is a rapidly progressive lesion,
such as, metastasis.
Extramedullary tumors grow in relation to
a nerve root. Chronic progressive radicular pain, especially at night,
may precede all other symptoms. The combination of pain associated
with myelopathy can progress for a long time by the patients'
ability to cope. Autonomic symptoms are delayed as the center of the cord
is involved late unlike the intramedullary tumors. Radicular pain may
simulate an angina at times.
Intramedullary tumors infrequently
progress slowly, & for a long time often with rather mild symptoms
and ill-defined pain. The mean distribution of symptoms prior to
operation are more than 4-5 years, ranging between 3 months & 11
years. Since these tumors often destroy structures near the centre of the
spinal cord, the crossing pain and temperature fibers are frequently
damaged and there is early involvement of bladder fibres. In the
classical case the tumor therefore presents with an early segmental
differential sensory deficit, later followed by long tract signs, with
subsequent weakness & wasting of musculature in the extremities.
However, the presenting symptoms do not necessarily suggest an
intramedullary process. Different degrees of paraesthesias, sensory loss,
motor deficits and atrophy of the extremity musculature atrophy are then
also encountered.
In children muscular weakness with gait
disturbances, back or extremity pain and urinary dysfunction are the most
common presenting symptoms. Up to 30% of the pediatric patients present
as spinal deformities. Spinal deformities, such as kyphosis or scoliosis,
when associated with pain often can be warning signs of a spinal cord
tumor.
Congenital
lesions are often signaled by mid-lying cutaneous markers such as
hemangiomas, a dural sinus tract, etc. Meningiomas, schwannomas,
and neurofibromas can be suggested by other neurocutaneous findings.
Signs
and symptoms with relation to site:
|
|
Spinal
cord
|
Conus
medullaris
|
Cauda
equina
|
|
Weakness
|
Symmetrical; profound
|
Symmetrical; variable
|
Asymmetrical; may be mild
|
|
Tendon reflexes
|
Increased
|
Increased AJ, decreased KJ
|
Decreased; asymmetrical
|
|
Plantars
|
Extensor
|
variable
|
Flexor
|
|
Sensory loss
|
Symmetrical; sensory level
|
Symmetrical, saddle
anesthesia
|
Asymmetrical; radicular
|
|
Micturition
|
Spared until late
|
Early involvement
|
May be spared
|
|
Progression
|
Rapid
|
Variable; may be rapid
|
Variable; may be slow.
|
|
An isolated conus tumor is not seen in practice, and at
presentation, it usually compresses the roots of cauda equina, and
presents as mixed or a cauda equina type of syndrome.
Diagnosis:
Despite
today's advanced imaging, it is vital to ascertain the site of the lesion
before requesting an investigation.
It
must be remembered that the spinal cord is much shorter than the
vertebral column and ends at the lower border of the L1 vertebral body.
Hence, the spinal cord segments are not situated opposite the
corresponding vertebrae.
There
is a progressive increase in the difference between the cord segments and
vertebral bodies from above downwards.
The
8 cervical segments extend from the foramen magnum to the upper C7
vertebral body.
The
12 dorsal cord segments lie opposite the D1 to the lower body of D9.
The
D4, D8, and the D12 cord segments lie opposite the D3, D6, and D9
vertebral bodies, respectively.
The
lumbar cord segments are opposite the D10, D11, and D12 vertebral bodies.
The
sacral and coccygeal segments therefore, lie opposite the L1 body.
Plain x-rays:
X-rays may alert a clinician. A widened spinal canal
(intramedullary tumor), bony changes (extradural and
extramedullary-intradural), and widened intervertebral foramen
(neurofibroma) warrant a further imaging. Paravertebral shadows may
suggest a malignancy.
Myelography:
The classical diagnostic
tool was myelography.
With an intramedullary tumor
almost invariably showing up as a widening of the cord shadow.
A filling defect
displacing the cord to one side is characteristic of extramedullary
lesion.
Serrated or brushlike
transverse filling defect suggests an extradural pathology.
MRI:
Magnetic resonance imaging (MRI) is the
most useful radiological study for evaluating the spinal canal and its
contents and is the imaging of choice. It
is useful both in defining the extent of disease and the possible
pathology involved and in evaluating the spinal cord and its surrounding
structures in multiple projections.
CT:
CT myelography is useful, when MRI is
not possible. The computed tomography (CT) scan is useful in particular
for defining bony abnormalities. CT scanning tends to offer very
little insight into intramedullary disease by itself. Its
delineation of soft tissue changes is vastly inferior to that noted by
the MRI scan. Occasionally, CT is combined with myelography, but
usually this does not give as clear an evaluation as that given by MRI.
|
|
|
|
|
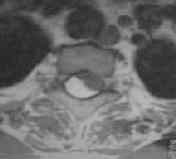
MRI-intramedullary lipoma
|
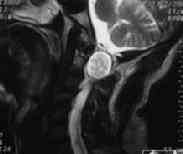
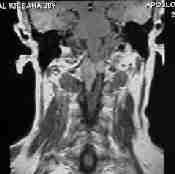
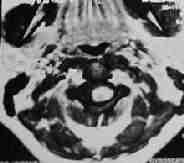
MRI-C1 extramedullary schwanoma
|
MRI-C1extramedullary schwanoma
|
|

|
|
|
|
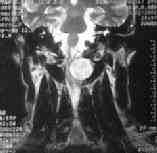
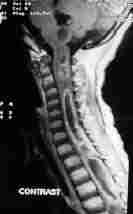
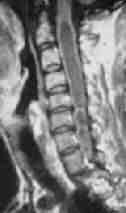
MRI-dumb bell neurofibroma
|
MRI-Foramen Magnum meningioma
|
MRI-intradural hemangioblastoma
|
|
|
|
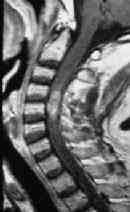
|
|
MRI-holocord
|
MRI-intramedullary ependymoma
|
MRI-intramedullary astrocytoma
|
Treatment:
Surgical excision is the treatment for
extramedullary tumors. Total excision along with involved dura in
case of meningiomas is possible and recommended. Sacrificing the nerve
root during total excision of neuromas may be justified.
The traditional treatment of intramedullary gliomas
has been biopsy followed by radiation therapy. However more & more
neurosurgeons have changed to aggressive treatment of these neoplasms.
The major reasons for this are the diagnostic and operative technical
developments that have taken place for the last few years. Thus, MRI increases
the accuracy of the diagnosis, and together with perioperative
ultrasonography it allows an exposure that minimizes bone removal while
maximizing tumor accessibility. the operative microscope and bipolar
coagulation marked the dawn of modern treatment of these lesions,
& the introduction of ultrasonic aspiration and surgical laser
dramatically modified the strategy in favor of aggressive surgical
treatment.
In experienced hands radical resection of ependymomas is now
possible, with good functional results. The same is true for
haemangioblastomas and cavernomas. Because of their clear demarcation
ependymomas and vascular tumors often can & should be totally
resected, without risk of increased morbidity.
With regards to malignant astrocytomas most surgeons agree
that surgery has only little impact on the clinical course. A less
radical intervention, to secure minimal surgical morbidity is therefore
usually recommended.
The surgical treatment of low grade astrocytomas is a bit
more controversial, with some authors advocating radical removal of the
tumor while others claim that total removal does not yield better outcome
compared to less aggressive resection.
Symptomatic
syrinx must be drained.
Post operative deformity, subluxation and instability is
reportedly common following extensive laminectomy in the young (under
18yrs of age), especially in the cervical region. Reportedly,
laminoplasty can prevent a deformity.
Some advise stabilization procedure as preventive measure.
Most surgeons advise a close follow up.
Radiation therapy for intramedullary tumors
has been controversial during the last decade. Clearly, radiation is
accompanied by a risk of spinal injury the functional tolerance of the
cord being 10 – 15% lower than that of the brain. Radiation sensitivity
increases with the length of the cord irradiated, the size of the daily
dose, and the total dose given. A total dose of 5000 rads given in 25
fractions over 5 weeks is usually considered acceptable.
Almost all studies support no indication for post operative
irradiation for intramedullary ependymomas and low grade astrocytomas.
With regards to high grade gliomas, for clinically
progressive lesions, and for tumors in which a substantial resection
cannot be achieved, most surgeons still agree
that radiation therapy is to be recommended, although with uncertain
results. Craniospinal irradiation is recommended for high grade
ependymomas due to the higher risk of tumor growth in the CSF pathways.
Radiotherapy is clearly of value in metastatic lesions.
Chemotherapy can be
considered in patients with progression of disease after radiation
therapy. There are a number of case reports and small series indicating
chemotherapy responses in pediatric and adult spinal cord astrocytomas.
Although astrocytomas of the cerebral hemispheres are not highly
responsive to chemotherapy, recent evidence has suggested that
astrocytomas with 1p loss may also be sensitive to chemotherapy.
Chemotherapy
can also be administered in ependymomas with progression of disease after
radiation therapy, since ependymomas demonstrate some responsiveness to
chemotherapy.
Prognosis:
The prognosis for extramedullary intradural tumors is good
following a total excision.
The reported results of treatment of intramedullary tumors
are still difficult to interpret and evaluate because of heterogenous
management strategies, small number of patients and short periods of
follow up. Clearly, most patients experience some neurological morbidity
in the immediate post operative period, deficits which in benign lesions
may improve within 3-6 months.
Studies suggest that the surgical outcome at follow up is
directly related to the patients’ pre operative status. Thus recovery
from a significant & long standing deficit rarely occurs.
Prognostic
factors for patients with spinal cord gliomas include histological grade
and duration of symptoms prior to diagnosis.
Recurrence
is almost always due to tumor growth at the original tumor site, although
the possibility of simultaneous tumor dissemination throughout the
neuraxis should be also considered, especially with high-grade tumors.
At present malignant astrocytomas of the spinal cord
are incurable lesions with a behavior that is very similar to that of the
histologically identical lesions found in the brain. Thus although
operation may result in palliation, malignant astrocytomas usually recur
within a year, with a fatal outcome in less than 2 years after
operation. The
overall 5-year survival for patients 30% with high-grade tumors.
The prognosis of low grade astrocytomas is of course
better, with some claims of an excellent long term prognosis. Most
surgeons are more guarded. The overall 5-year survival is 70-90%.
The outcome following surgery of intramedullary ependymomas
is more gratifying. Radical removal can usually be achieved, and if so
tumor recurrence is very unusual. Overall survival of series of patients
with low-grade ependymomas of spinal cord are in the range of 85% 5-year
survival. Survival rates are even higher in patients with myxopapillary
ependymomas and are significantly lower in patients with anaplastic
ependymomas.
|