|
They
are the most common benign tumors of the brain. Dural endothelioma,
fibroma, sarcoma, epithelioma, and fungoid tumors of the dura are
some of the older names that existed until Cushing established
the term meningioma in his Cavendish lecture of 1922. About 90 per
cent of all CNS meningiomas are intracranial.
Incidence:
The
incidence ranges around 20 per cent of all brain tumors. In India,
the incidence ranges from 9-15 per cent of all intracranial
neoplasms in various series. The incidence seems to be higher in
Africa, at 24-38 per cent. Meningiomas most commonly occur in the
middle decades of life. In India, these tumors have been
reported to occur predominantly between the third and the fifth
decades, with a peak in the fourth decade. Western literature
suggests maximum prevalence of meningiomas between the fourth and the
sixth decades. Meningiomas are more commonly encountered in women
than in men. There is no sex preference in older patients.
Familial incidence of meningiomas, usually
multiple, is largely found in association with central
neurofibromatosis (NF-2).
Meningiomas
are rare in children, they form 0.4-0.6 per cent of all intracranial
neoplasms in childhood. About two per cent of all meningiomas
occur in childhood and adolescence. Meningiomas in children are more
commonly malignant and often of the haemangiopericytic and papillary
type. A higher prevalence of cystic meningiomas has also been
reported in children. The other distinctive features of meningiomas
in children are i) no sex preference, ii) a particularly high
incidence of intraventricular tumors, and iii) significant
association with neurofibromatosis.
Etiology:
Though
the origin of a meningioma, like of any other neoplasm, is uncertain,
some antecedent factors have been implicated in the initiation and
growth of meningiomas.
Trauma to the head has been blamed for a
long time as an important contributory cause. Though in the majority
there are no morphological signs of trauma at the site of the tumor,
in some cases the tumor had arisen under a fracture, from an area of
dural scarring or even from a retained foreign body intracranially.
Despite these conflicting reports, there is enough evidence to
suggest that at least some cases of intracranial meningiomas are
initiated by head injury.
Chronic irritation, from an
ossified subdural hematoma or tubercular pachymeningitis, was
incriminated in the past as a causative factor. However, it is
not considered relevant. Papova virus large T-antigens have been
demonstrated in a high percentrage of meningiomas. Herpes virus large
T- antigens seem to induce meningioma growth. Recent technical
developments have allowed the identification of small pieces of viral
proteins in human tumors, including meningiomas. Although it is
not possible to say whether these viral genes or vital proteins are
the etiological gents in meningiomas, their presence is an important
step in establishing a relationship between the virus and meningioma.
Irradiation induced meningiomas have
appeared following high dose irradiation for intracranial growths and
low dose radiation to the scalp for fungal disease and occasionally,
for a vascular nevus. The onset of tumor formation can be 12-27 years
later. While the majority of the radiation induced tumors following
high doses were thought to be sarcomas, a recent review of the world
literature suggested, radiation induced meningiomas are at least five
times more numerous than gliomas or sarcomas. Some of the unique features
of radiation induced meningiomas are 1)The neoplasm lies below and
often invades the atrophic scalp with alopecia, 2)The tumor occurs in
a much younger age group; the greater the radiation dose, the shorter
the latency and the younger the patient’s age at presentation, 3) No
female predominance, 4) A calvarial location abutting against the
sagittal sinus, 5) Multiple tumors are more common (25-29 per cent),
6) Recurrence following excision is common.
The
carcinogenic effect of thorium dioxide has been blamed in the genesis
of some meningiomas.
Chromosomal
abnormalities in meningiomas are now well established and
probably more consistently seen than in any other tumor except
chronic granulocytic leukemia. In approximately 80 per cent of
the tumors analyzed there is a loss of heterozygosity on at least one
chromosome 22 DNA marker. The frequency and consistency with
which monosomy 22 appear has led to the postulation of a uniform
pathogenetic mechanism and it has been hypothesized that with the loss
of genetic material on chromosome 22, a previously suppressed
oncogene is probably unmasked. The role of SV-40 virus in meningiomas
is disputed by some.
Increased
incidence of meningiomas, usually multiple, are associated with
neurofibromatosis 1 & 2. Patients with von Recklinghausen’s
disease develop meningiomas at an early age; 19-24 per cent of
adolescents with meningiomas have neurofibromatosis. The other
evidence of heredofamilial occurrence is the association of
meningioma with Von Hippel-Lindau disease.
Hormonal
association
is indicated by the greater incidence of meningiomas in females, its
increase in size related to pregnancy and the luteal phase of the
menstrual cycle, and the documented association between meningioma
and breast carcinoma in the same patient. However, the
existence of sex-specific hormone receptors in meningiomas has long
been a controversial issue.
Despite
the frequent inconsistencies, binding assay techniques in meningiomas
suggest: (1) high levels of progesterone receptors, (2) moderate
concentration of androgen receptors, and (3) an equivocal report
about the status of estrogen receptors. The recent cloning of
complementary deoxyribonucleic acid (cDNA), encoding human estrogen,
progesterone and androgen receptors has facilitated the direct
investigation of hormone receptor gene expression without the
limitation of variations in binding assay interpretation. The
coexpression of androgen and progesterone receptor messenger
ribonucleic acid (mRNA) and protein product have been reported in few
meningioma. Estrogen receptors in mRNA expression were not
detected.
Pathology:
Almost all
meningiomas are intradural. However, extradural meningiomas, both
cranial and spinal, have been reported. Meningiomas may be
globular in form or flat.
|
The globular tumors may be
rounded, ovoid or lobulated and usually have a relatively small
dural attachment. Globular tumors are usually smoothly lobulated
and well encapsulated with the result that
characteristically, the
adjacent brain is not invaded and an intact pial covering is
usually present.
On the contrary, the flat
tumors commonly referred to as meningioma en-plaque, are less well
encapsulated with a tendency to involve the pia as well as the
overlying bony structures. They are attached over a
relatively broad area of the dura.
Meningiomas have a tendency
to invade the dura and its venous sinuses and may grow through the
skull into extracranial tissue.
|
|
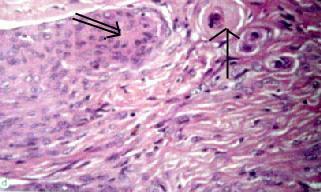
|
|
Meningothelial
meningioma (H&E): meningothelial cells and
fibrous areas with attempts at whorl formation (doublearrow)
and psammoma bodies(arrow).
|
|
A majority
of the tumors are solid, but areas of cystic degeneration or a
predominantly cystic tumor may occur. Granular or patchy
calcification may occur, especially in the psammomatous variety and
occasionally, the tumor may be totally calcified. Peritumoral
brain edema is a common feature and suggests an aggressive nature.
Multiple
meningiomas are more commonly encountered in the pediatric population
(11 per cent), in the elderly (up to 20 per cent) and in patients
with neurofibromatosis (20 per cent). These tumors can occur at any
location within the cranium, and the association of cranial and
spinal meningioma is rare. Multiplicity may result from a
multicentric origin of the tumor or from dissemination of tumor cells
by CSF during surgery.
Occassional
association with aneurysms and AVMs and gliomas has been reported and
considered coincidental.
Association
with other intracranial neoplasms, such as acoustic neurinoma, in the
absence of neurofibromatosis, is extremely rare.
Cystic
changes may, occasionally occur at the periphery of a meningioma
(peritumoral) or inside the tumor (intra-tumoral). Intratumoral
cysts arise from degeneration, hemorrhage or necrosis.
Peritumoral cysts arise from adhesions and accumulation of protein
containing CSF, reactive gliosis, fibroblastic proliferation in the
final stage of peritumoral oedema or rarely as an exudate from the
tumor surface. From a surgical point of view, peritumoral
cystic meningiomas present greater difficulties and unless every
effort is made to excise not only the mural nodule, but also the cyst
wall with the help of an operating microscope, recurrence is likely
to occur. The cystic variety is more commonly encountered in males,
in children and in the supratentorial compartment. Cystic changes in
a meningioma may have a serious connotation as eight per cent of
cystic meningiomas are reported to be malignant and 12 per cent are
reported to be angioblastic, probably hemangiopericytic.
Meningiomas
arise from the arachnoid cells. The arachnoid cell has a polyblastic
character and is functionally multipotential. This results in
different histological and cytological variations of meningiomas.
Classification
of the meningiomas has been changed several times.
The WHO
classification of meningioma (2000):
|
Classification
|
|
Features
|
|
Meningothelial (grade I)
|
|
Fairly uniform polygonal
cells with indistinct cytoplasmic borders arranged in sheaths or
medium size globules.
|
|
Fibroblastic (grade I)
|
|
Spindle shaped cells in
a dense collagen matrix.
|
|
Transitional (grade I)
|
|
Mixed of above types.
|
|
Psammomatous (grade I)
|
|
Cells are more elongated
and separated . Form whorls which by degeneration forms
Pssmmonian bodies (concentric laminas of degenerated cells have a
concentration of calcium salts).
|
|
Angiomatous (grade I)
|
|
Abundant sclerosing
blood vessels.
|
|
Microcystic (grade I)
|
|
Cells have stellate and
vacuolated cytoplasm with long cytoplasmic processes.
|
|
Secretory (grade I)
|
|
Epithelial differentiation
of meningothelial cells resulting in the production of hyaline
inclusions.
|
|
Lymphoplasmacyte-rich
(grade I)
|
|
Lymphoplasmacytic
infiltration in the meningothelial component of the tumor.
|
|
Metaplastic (grade I)
|
|
Meningothelial cells
with differentian into spindle cells.
|
|
Clear cell (grade II)
|
|
Mixture of
clear cells and meningothelial cells.
|
|
Chordoid (grade II)
|
|
Spindle or
epitheloid cells disposed in chordoma-like clusters and cords in a
myxoid matrix.
|
|
Atypical (grade II)
|
|
More
cellularity and cytologic atypia than grade I tumors.
|
|
Papillary (grade III)
|
|
Papillary pattern with
few anaplastic features.
|
|
Rhabdoid (grade III)
|
|
Abundant
eosinophilic cytoplasm resembling rhabdoid tumor.
|
|
Anaplastic (grade III)
|
|
Has a high cellularity,
brain invasion, frequent mitosis, invasion of the blood vessels and
necrosis.
|
|
The meningotheliomatous
meningioma is the commonest histological type, though some report
them to be less common. Recent advances in pathology include the recognition
of cystic types, evaluation of proliterative activity and the use
of markers in the evaluation of the aggressiveness of meningiomas
in the delineation of malignant phenotypes.
Sites of
Origin:
|
|
Approximately 90 per cent of the intracranial
meningiomas are supratentorial. In the cranial cavity as a
whole, the anterior half is involved far more frequently than the
posterior half.
The most common sites are the convexity,
parasagittal, falx, and sphenoid ridge, together making up 60 per
cent of intracranial meningiomas.
Parasagittal
Meningiomas arise from the arachnoid villi of
the superior sagittal sinus and often involve the adjacent
convexity dura and falx. Nearly 50 per cent invade the sinus,
50 per cent get secondary attachment to the falx and 25 per cent
are bilateral. Hyperostosis is associated with 25 per cent of
these tumors and is a valuable pointer to their diagnosis.
Falcine
meningioma arises from the falx cerebri or inferior sagittal
sinus and may rarely invade the superior sagittal sinus. It
is usually completely concealed by the overlying cerebral cortex
and does not cause bony changes. About 50 per cent of the
tumors grow through the falx to become bilateral. Falx
meningiomas are about five to seven times less common than
parasagittal meningiomas.
|
|
|
Site
|
Distribution
|
|
Convexity
|
34%
|
|
Parasagittal
|
22%
|
|
Sphenoid
ridge
|
17%
|
|
Lateral
ventricle
|
5%
|
|
Cerebellar
convexity
|
5%
|
|
Tentorium
|
4%
|
|
Tuberculum
sella
|
3%
|
|
Orbital
|
2%
|
|
Cerebello
pontine angle
|
2%
|
|
Olfactory
groove
|
3%
|
|
Foramen
magnum
|
1%
|
|
Clivus
|
1%
|
|
The
distribution of parasagittal and falx meningiomas along the longitudinal
axis is about 20, 50 and 30 per cent in the anterior, middle and
posterior third, respectively.
Convexity Meningiomas may occur
anywhere over the convexity of the cerebrum. Convexity tumors may
cause erosion of the overlying skull and may come to lie under the
scalp.
Olfactory meningiomas may arise
from the anterior part near the crista galli, from near the
cribriform plate or the planum sphenoidale. These tumors can be
silent for a long time. Growing posteriorly, these tumors
compress the optic nerve and chiasma leading to unilateral blindness
or bitemporal hemianopia with optic atrophy. With the rise in
intracranial pressure, there may be papilledema in the opposite eye
and Foster Kennedy syndrome may be seen. Further extension posteriorly
puts pressure on the hypothalamus and pituitary gland. By this time,
the ICP rises to cause obvious features of raised ICP. It is
not unusual, even today, to see large olfactory groove meningiomas
presenting with blindness and raised ICP. Rarely, by eroding
through the orbital roof or the cribriform plate, the tumor may cause
proptosis.
Suprasellar Meningiomas include
meningiomas arising from the tuberculum sellae, planum sphenoidale,
diaphragma sellae and/or anterior clinoid process in close proximity
to the optic chiasma, displacing it posteriorly and superiorly and
stretching it. They may extend into the orbit, paranasal sinuses,
cavernous sinus, sella, infratemporal fossa, and posterior
fossa.
Medial Sphenoid Wing (clinoidal) Meningioma can be
divided into two general categories. These are: 1) globular and
2) diffuse or enplaque. The globular meningioma grows en mass
from the anterior clinoid and medial sphenoid, involves the ICA and
MCA to variable degrees and displaces or engulfs the optic nerves,
chiasma, and optic tracts and compresses the adjacent frontal and
temporal lobes. The second variety grows diffusely from a
similar area with involvement of the cavernous sinus and often
without symptoms of an intracranial mass. As they grow bigger, the
branches of the fifth, fourth and sixth cranial nerves may be
affected.
Middle-third Sphenoidal Wing (Alar) Meningiomas arise from
the middle third of the sphenoid wing in relation to the superior
orbital fissure (SOF) and the anterior portion of the middle cranial
fossa (MCF). Growing posteriorly, it indents the temporal lobe.
Lateral Sphenoidal Wing (Pterional) Meningiomas with a
minimal reaction in the sphenoid ridge is more common than the en
plaque variety. The tumor occupies the middle cranial
fossa, may extend into the anterior fossa and attain a large size
before symptoms become obvious. Meningioma en plaque, is
uncommon and behaves in a peculial fashion, in that the tumor spreads
along the meninges as a plaque causing an intense bony
reaction. There is hyperostosis of the pterion as well as the
lateral half of the lesser wing of the sphenoid. Tumor may also
be present in the lateral and posterior orbit and may involve the
optic canal.
Cavernous Sinus Meningiomas may be
classified into (a) the confined and (b) the extensive group.
The confined tumors are small tumors that involve the cavernous sinus
and Meckel’s cave, the middle fossa or the sella turcica. The
extensive tumors include petroclival, medial sphenoid wing and infratemporal
tumors that involve the cavernous sinus. These are generally known to
be slow growing tumors, though the natural history is not
clear.
Middle Cranial Fossa Meningiomas may arise
anywhere in the middle cranial fossa or may extend into it from the
anterior surface of the petrous temporal bone or lateral surface of
the cavernous sinus. Paresthesia or numbness of the face may be
present and lacrymation may be impaired. The tumor indents the
undersurface of the temporal lobe and may remain asymptomatic for a
long time. The foramen spinosum and the middle meningeal artery are
considerably enlarged.
Posterior Fossa Meningiomas constitute
8-12 per cent of all intracranial meningiomas and 7-12 percent of all
posterior fossa tumors. They are, conventionally, classified
according to the site of dural attachment as follows: 1) cerebellar
convexity, 2) tentorium, 3) posterior surface of the petrous bone, 4)
clivus, 5) foramen magnum, and 6) fourth ventricular (tela choroidea.
The posterior surface of the petrous bone is the commonest site of
attachment (42 per cent) in posterior fossa meningiomas and these
meningiomas constitute 6-8 per cent of all cerebellopontine angle
tumors. The other characteristic features of these tumors are a broad
base towards the petrous bone and associated hyperostosis or erosion
of the petrous.
Meningiomas
arising from the clivus are attached at any of the several sites
along the petroclival borderline where the sphenoid, petrous, and
clival bones meet. The zone of adherence to the dura is commonly wide
and overlaps two or more of these sites. Moreover, almost all
these tumors have wide tentorial occupation. Foramen magnum
meningiomas are the commonest tumors of the foramen magnum.
Tentorial Meningiomas may arise
from any location on the tentorium and account for two to three per
cent of all intracranial meningiomas. Tentotial meningiomas may
grow upwards into the posterior fossa or in both directions.
Nearly 20 per cent have significant supra and infratentorial extensions.
Torcular meningiomas
have, as part of their dural base, the dura forming the torcular,
i.e., they arise from, invade, or are attached to a wall of the
torcular itself. These tumors represent about one per cent of
intracranial meningiomas. True torcular meningiomas are usually
bilateral, based on the torcular. When there is only unilateral
extension from the torcular it is usually a lateral tentorial
meningioma which has got secondary attachment to the torcular.
Often, these tumors have both infra and supratentorial extension
bilaterally.
Intraventricular Meningiomas constitute
1-1.7 percent of intracranial meningioma and usually arise from the
choroids plexus of the lateral ventricle, but may occur rarely in the
third or fourth ventricle. The lesion is more frequent in the left
lateral ventricle in middle aged women, but has been well documented
in children. 60-94 per cent of the lateral ventricular meningiomas
arise from the choroid plexus at the trigone. Intraventricular
meningiomas are thought to arise from arachnoid tissue, which is
carried with the choroid plexus as the ventricular system
invaginates.
Intra-temporal meningiomas are rare.
The usual sites are near the jugular foramen, the internal auditory
meatus, the region of the geniculate ganglion and the sulci of the
superficial petrosal nerves. Jugular foramen meningiomas are often
clinically indistinguishable from glomus jugulare tumors. Occurring
inside the temporal bone, these tumors often infiltrate the
surrounding bone. Some of these cases have en plaque tumors
over the petrous.
Orbital Meningiomas are
discussed elsewhere.
Extracranial Meningiomas: ExtracraniaL
(excluding spinal) meningiomas constitute one per cent of all
meningiomas and can be classified into four groups. These are as
follows:
Group 1: Arising from intracranial dura and
extending extracranially. This is the most common type of
extracranial meningioma. Extracranial extension of intracranial
meningiomas is described in four principal sites: 1) the orbit (7.5
per cent), 2) the outer dipole and scalp (six per cent), 3) the upper
respiratory tract (2.5 per cent) and 4) the parotid region and
infratemporal fossa (1.25 per cent). Most parapharyngeal
meningiomas are related to the cranial nerves, particularly 7th,
9th, 10th, 11th and 12th.
Group 2: Head and neck extracalvarial
meningiomas: Extracranial meningioma, in the absence of an
intracranial mass, but associated with hyperostosis of the underlying
skull, osteolytic changes and intra-osseous tumor infiltration have
been described in the outer surface of the frontal, temporal and
parietal bones. A primary intra-osseous location without
underlying dural involvement is very rare. Arachnoidal cell clusters
normally found at the level of the internal auditory meatus(IAM),
jugular foramen, geniculate ganglion, roof of the eustachian tube or
in association with the greater or lesser petrosal nerves, may represent
the cells of origin of temporal bone meningiomas.
Group 3: Ectopic meningiomas not associated
with the craniospinal meninges: An ectopic meningioma was first
reported by Winkler, who, in 1904, described a case of paravertebral
subcutaneous meningioma in a 10 year old girl. Other ectopic
sites reported are the glabella, pterygopalatine fossa, intraoral,
nasal cavity, paranasal sinuses, parotid gland, neck, cutaneous areas
of the scalp, the face, mediastinum, lung, little finger, brachial
plexus, lung, and adrenal gland
Group 4: Metastatic meningiomas: Metastases
from a meningioma could be extraneural or through the CSF
pathways. A total of 16 cases with CSF spread have been
reported. Eleven cases had features of malignancy in the
original neoplasm and seven cases had associated extraneural
metastases. In five cases both the original tumor and the
deposits preserved their benign character. Though tumor seeding
at operation might have been the explanation in two, no surgery was
performed in three and these are examples of spontaneous
leptomeningeal metastases.
Extraneural metastasis is more
frequent than CSF dissemination. The hemangiopericytic and papillary
variant had a greater propensity to metastasize. Seventy per cent of
patients recorded to have had a metastasizing meningioma have been
subjected to previous craniotomy. However, spontaneous
hematogenous metastases have been reported and have been attributed
to invasion of the superior sagittal sinus, cavernous sinus and its
perineural lymphatics. Nearly one-third of all the metastases
were observed in the lung and the other common metastatic sites were
liver (19 per cent), lymph node (12 per cent) and bone (nine per
cent). The rare sites reported are the mediastinum, kidney,
thyroid and parotid.
Clinical features:
The
clinical presentation of a meningioma is classically with seizures,
hemiparesis, visual field loss, aphasia or other focal
symptoms. The clinical presentation depends on the location of
the meningioma. Most meningiomas are slowly growing lesions and
symptoms and signs will frequently develop very slowly. Finally an
increasing number of meningiomas are asymptomatic and are incidental
findings.
Convexity meningiomas: They may
exist for a long time without symptoms or they may lead to early
irritation of the cerebral cortex, resulting in partial or
generalized epilepsy, especially if located adjacent to the central
sulcus. The tumor makes a bed for itself on the surface of the
brain.
Parasagittal and falx meningiomas:
Anterior-third meningiomas, located between the crista galli and the
coronal suture, have a more insidious onset and often attain a large
size before diagnosis. Headache is the predominant symptom and
may be present for years followed by gradually progressive impairment
of memory, intelligence and personality changes. Generalized epilepsy
is a presinting symptom in 25-50 per cent of patients. Ataxia,
tremor and ipsilateral facial pain may, occasionally, accompany a
large meningioma in this location and thus may be misdiagnosed a
posterior fossa tumor. Tumors in the middle-third, from the coronal
suture to the lamboid suture, classically present with contralateral
focal motor sensory epilepsy followed by progressive weakness of the
lower limb. These tumors are detected at an early stage because
of focal epilepsy. Bilateral tumors may, occasionally, give
rise to bilateral disturbances and rarely paraplegia which may be
wrongly attributed to spinal pathology. Tumors in the
posterior-third, between the lamboid suture and the torcular
Herophili, may present with features of raised ICP alone. The
only characteristic sign, a homonymous field defect, either
quadrantanopic or hemianopic, may not be noticed by the
patient. Epilepsy is uncommon.
Olfactory meningiomas: Headache is
the most common presenting symptom. Though anosmia occurs in 85-90
per cent of cases, it is rarely the initial or presenting
symptom. As these tumors grow in size, symptoms of pressure on
the frontal lobe may be apparent. Mental symptoms often lead
the patient to seek treatment from a psychiatrist. While
inferior tumors may cause excitement or restlessness, pressure over
the convexity of the frontal lobe may lead to indifference and
apathy. The more anterior tumors cause a central scotoma and
papilledema. Growing posteriorly, these tumors press on the
optic nerve and chiasma leading to unilateral blindness or bitemporal
hemianopia with optic atrophy. With the rise in intracranial
pressure, there may be papilledema in the opposite eye and Foster
Kennedy syndrome may be seen. Further extension posteriorly puts
pressure on the hypothalamus and pituitary gland. By this time,
the ICP rises to cause obvious features of raised ICP. It is
not unusual, even today, to see large olfactory groove meningiomas presenting
with blindness and raised ICP. Rarely, by eroding through the
orbital roof or the cribriform plate, the tumor may cause proptosis.
Suprasellar meningiomas: Meningiomas
arising from the tuberculum sellae, planum sphenoidale, diaphragma
sellae and/or anterior clinoid process are conventionally grouped
under suprasellar meningiomas. As these tumors arise in close
proximity to the optic chiasma, displacing it posteriorly and
superiorly and stretching it, visual symptoms are early and common, leading
to earlier detection than olfactory groove meningiomas. Ninety
to ninety nine percent of the patients complain of either monocular
(55 per cent) or binocular (45 per cent) visual loss. The other
common symptoms are headache, epilepsy and mental changes. The
presence of bitemporal hemianopic field defects in the presence of a
normal sized sella should suggest the possibility of a suprasellar
meningioma. However, in the early stages vision may be affected
in only one eye. Pituitary hypofunction is uncommon and is found in
only 4-13 per cent of these patients.
Medial sphenoid wing meningiomas: They
present with slowly progressive ipsilateral visual impairment with or
without diplopia. Diplopia secondary to oculomotor paresis is
more common in the diffuse variety. As they grow bigger, the
branches of the fifth, fourth and sixth cranial nerves may be
affected. There may be proptosis because of either obstruction
of the anterior end of the cavernous sinus or draining orbital veins.
The other presenting symptoms may be headache, epilepsy or
psychiatric disturbances. Pressure on the hypothalamus may
become apparent as the tumor grows upwards and medially.
Middle-third Sphenoidal Wing (Alar) Meningiomas:
Proptosis is a frequent early symptom. The tumor usually attains a
large size before it is diagnosed. Growing posteriorly, it
indents the temporal lobe and thus uncinate fits or other symptoms of
complex partial epilepsy may become manifest.
Lateral Sphenoidal Wing (Pterional) Meningiomas:
They
present with a very slowly progressive unilateral, painless,
non-pulsatile proptosis and fullness under the temporalis
muscle. Some patients complain of a dull pain over the temple
and mild local tenderness.
Cavernous Sinus Meningiomas: These are
generally known to be slow growing tumors, though the natural history
is not clear. The symptoms are of long duration and include
retro-ocular pain, mild exophthalmos and double vision due toe VI
nerve involvement. Anesthesia in the distribution of the first
division of the V nerve may be seen. The confined tumors
generally cause more symptoms than the extensive tumors.
Middle Cranial Fossa Meningiomas: Paresthesia
or numbness of the face may be present and lacrymation may be
impaired. The tumor indents the undersurface of the temporal
lobe and may remain asymptomatic for a long time.
Posterior Fossa Meningiomas: Depending
on the site of origin, the tumor causes cerebellar, cerebellopontine
angle or brainstem syndromes with multiple cranial nerve
palsies. Features of raised ICP appear earlier than in
supratentorial meningiomas.
Intratemporal meningiomas: They
present with otological problems; symptoms of ear discharge,
mastoiditis, polyps or granulation tissue. Hearing impairment
and facial nerve paresis often develop. These patients invariable
have some degree of lower cranial nerve paresis. It is not
uncommon for these patients to present with a submandibular swelling
or a swelling in the posterior pharyngeal wall. When the lesion
extends into the posterior fossa, cerebellar signs may become
prominent.
Intraventricular meningiomas usually
present with symptoms of increased ICP; frontal lobe signs may be
present.
Hemorrhage in
meningiomas has been more frequently reported in tumors with a
parasagittal or convexity location, and more often in the malignant
or angioblastic varieties. However, an apoplectic presentation is
much less common. Other reported intracranial vascular events related
to meningiomas are rare and are secondary to either dural venous sinus
occlusion manifesting as pesudotumor cerebri or arterial
occlusion.
|
Imaging:
Plain X-ray: Abnormalities
in the skull films of patients with intracranial meningiomas have
been variously reported as 36-77.5 per cent in the literature.
Relatively less vascular meningiomas may cause a deposit of
minerals in the bone, leading to an increased.
density and thickening or hyperostosis, the
commonest primary change. Sclerosis of the bone does not
necessarily represent bone invasion, however, sclerosis of the
outer table of the skull as well as spiculation of the bone suggest
penetration of the bone by the tumor. Hyperostosis may be
focal near the attachment of the tumor to the meninges, the bony
projection resembling a osteoma. In other cases there is a diffuse
thickening of the bone. This process is particularly well marked in
the region of the sphenoid wing. Hyperostosis is reported in 15-44
per cent of adults and 10 per cent of children with meningiomas.
A highly vascular tumor nears the skull causes
rarefaction and bone absorption. Lytic skull defects suggest
penetration of the bone by the tumor and occasionally, the tumor
may protrude through a defect in the skull and lie under the
scalp. Meningiomas associated with a lytic destructive
reaction are reported to be biologically more aggressive and are
more likely to recur. Osteolytic changes are seen in 12 per cent of
adults and nine per cent of children with meningiomas.
Increased vascular markings are reported in
4-20 per cent of adults and four per cent of children: these could
be either focal areas of increased vascularity at the tumor
attachment producing a sinusoidal appearance in the bone, or an
enlargement and tortuosity of meningeal vascular channels.
Asymmetric unilateral enlargement of meningeal vascular channels
and an ipsilateral dilated foramen spinosum are highly suggestive
of a meningioma.
Tumor calcification has been reported in 9-20
per cent of adults and 13 per cent of children. Psammoma
bodies, stromal calcification and rarely, tumor ossification result
in calcific changes.
Magnetic Resonance Imaging: It is the
imaging of choice. On the unenhanced MR meningiomas are often
isointense with brain on T1 and T2 weighted images.
Extra-axial mass effect suggested by white matter buckling, a rim
of CSF around the mass, a pial vascular rim and a shorter T2 of the
mass are described as characteristics of meningioma.
Gadolinium enhanced MR suggest that MR is better suited for
identifying the extra-axial location of the tumor, the broad
contact with the meninges, the tumor capsule and meningeal contrast
enhancement adjacent to the tumor, i.e., the meningeal sign.
CT is, however, superior in demonstrating calcification and
atypical tumor density. Both methods provided nearly equal
results in demonstrating mass effect, hyperostosis and contrast
enhancement. Contrast enhanced MR (CEMRI) is particularly
superior in the diagnosis of meningiomas of the skull base,
posterior fossa and high convexity.
A thickened and enhanced dura, variously
called ‘dural tails’ and ‘the meningeal sign’ can be identified
adjacent to some meningiomas. Dural tails are considered as signs
of tumor infiltration along the dura, as proven by
histopathological examination. Incomplete excision of this
extensive dural tail may lead to recurrence.
MR spectroscopy may also
be used for metabolic or functional studies of meningiomas.
Computed Tomography (CT): Plain and
contrast enhanced (CE) CT scans are positive in 96 per cent and
diagnostic of a meningioma in 90 per cent of cases. Meningiomas are
dura based extra-axial mass lesions with broad contact with the
meninges. On the plain CT 75 per cent of tumors are hyperdense and
14.4 per cent are isodense. They are often multi-lobulated and
smooth in contour, adjacent to dural structures and may be
calcified in some areas. Intravenous X-ray contrast enhances
meningiomas uniformly and brightly. In about 15% of cases
atypical patterns such as, necrosis, cyst formation or hemorrhage
is found. Indistinct margins, marked edema, mushroom like
projection from the tumor, invasion deeply into the brain, and
heterogeneous enhancement all suggest aggressive types. Peritumoral
brain edema is seen in 60-75 per cent of meningiomas. The edema
around the tumor is associated with aggressive tumors, and is
either a result of a break down of the blood-brain barrier or a
secretion of the tumor itself. The association of bone changes like
hyperostosis or a lytic area at the tumor base helps in the diagnosis
of meningiomas. Peritumoral low attenuation may also be caused by
demyelination, entrapped ventricular CSF, a subarachnoid cyst or
peritumoural cyst, or the co-existence of a glioma.
3D CT angiography and MR angiography
delineates the encasement and displacement of the intracranial
vessels and is as good as angiography.
Angiography: The availability of CT and
MR has considerably decreased the indications for angiography in
the diagnosis of brain tumors. Still, angiography is often
preferred by the surgeons in the management of parasagittal, falx
and basal meningiomas and also to study the encasement of major
intracranial arteries, the patency of the dural sinuses and the
venous anatomy (e.g. cortical venous drainage to the sagittal sinus
in parasagittal or falx meningiomas and the anatomy of the vein of
Labbe in petroclival meningiomas), for planning the operative
approach. Occasionally, angiography may be helpful in the
diagnosis of a meningioma in an atypical case, by demonstrating
external carotid supply to the tumor, although other primary tumors
of the meninges and metastatic tumors of the calvarium may also
have an external carotid supply. Currently angiography is more
often used in the evaluation of the feasibility of
embolisation.
Positron emission tomography (PET)
with F-2-fluorode-oxyglucose has been used to evaluate small
changes in CT or MR imaging to determine whether these were
recurrent tumours. Research in meningioma receptor ligands
for PET scans may reveal additional information of the tumor
biology useful for the preoperative assessment.
MANAGEMENT:
Surgery:
The objective of surgery is total removal of
the meningioma, including the dural attachment and bone that is
involved by the tumor. The completeness of surgical removal
is the single most important prognostic factor. However, when total
removal entails unacceptable risks of morbidity or mortality, it is
prudent to be satisfied with subtotal excision. Sound
judgment in choosing the best treatment depends on a high level of
clinical acumen, for the best treatment is that which is best for
the patient, not necessarily what is best for the tumor. The
factors having a direct bearing on the surgery of meningiomas are
its location, vascularity, size and consistency.
Preoperative
embolization in the external carotid system, though helpful in
reducing bleeding and shortening the operation time; is not without
the hazard of inadvertent reflux of emboli into the internal
carotid system causing cerebral infarction. Surgery should follow
within 24 hours of embolization. As an alternative, I prefer to
expose the carotids at the neck for temporary occlusion in highly
vascular lesions.
Preoperatively, all patients are
prophylactically put on anticonvulsants. I prefer to give
intravenous dexamethosone (0.5 mg/kg body weight stat followed by
4mg 6 hourly) the day before the surgery along with H2
antagonist.
|
|
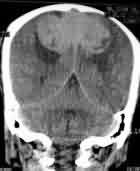
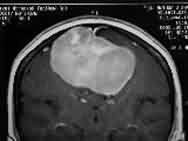
|
|
|
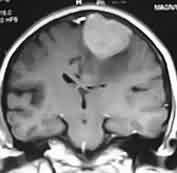
Bifalcine
meningioma-MRI
|
Falx meningioma-MRI
|
|
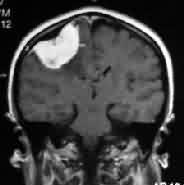
|
|
|
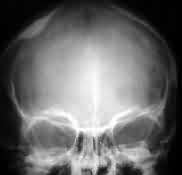
Convexity meningioma
with hyperostosis- MRI
|
Convexity meningioma
with hyperostosis- X-ray
|
|
|
|
|
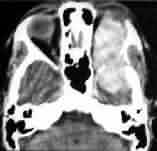
Orbital meningioma-CT
|
Parasagittal
meningioma-MRI
|
|
|
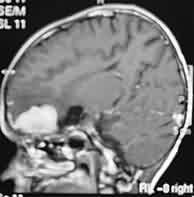
|
|
Meningioma with
Associated pituitary adenoma-MRI
|
Olfactory groove
meningioma-MRI
|
|
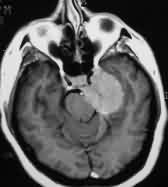
|
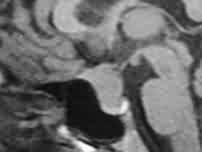
|
|
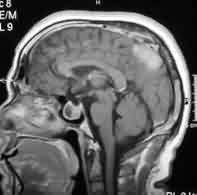
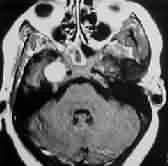
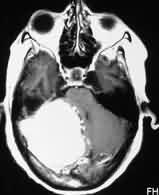
Petrous
meningioma-MRI
|
Suprasellar
meningioma-MRI
|
|
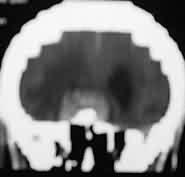
|
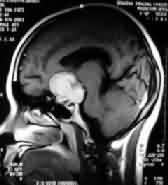
|
|
Tuberculum sella
meningioma-CT
|
Diaphragm sella
meningioma-MRI
|
|
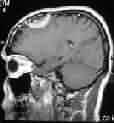
|
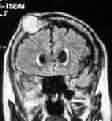
|
|
Meningioma with skull
infiltration-MRI
|
Intradiploic
meningioma-MRI
|
|
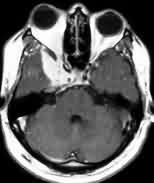
|
|
|
Sp. Wing en plaque
meningioma-MRI
|
Meningioma extending
through foramen ovale -MRI
|
|
|
|
|
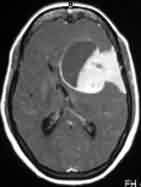
Intraventricular
meningioma-MRI
|
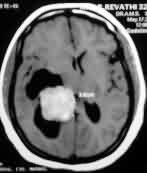
Cystic meningioma
with dural tail-MRI
|
|
|
|
|
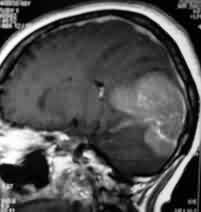
Tentorial
meningioma-MRI
|
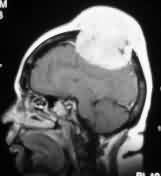
Torcular
meningioma-MRI
|
|
|
|
|
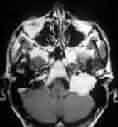
Hemangiopericytoma-MRI
|
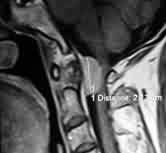
Jugular foramen
meningioma-MRI
|
|
|
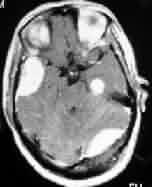
|
|
F.M
meningioma-pre op-MRI
|
Multiple
Meningiomas -MRI
|
|
At surgery, the head is
secured higher than the level of the heart and without compression of
the neck veins.
As a general
rule, the site of incision is positioned as the highest area in the
scalp to maximize the accessibility of the tumor. Free bone flaps are
generally preferred over the osteoplastic flap. Hyperostosis and
infiltration of bone by the tumor increases the difficulty during
elevation of the flap. Bleeding from the bone can be most troublesome
as the saw cut is being made and also when the bone flap is being
elevated. Vigorous and frequent application of bone wax and rapid
turning of the flap help to minimize bleeding.
Fortunately,
a layer of arachnoid usually separates the meningiomas from the
brain, cranial nerves, and blood vessels. By accessing and staying
within this surgical plane, the chances of neural and vascular injury
are minimized. Early extensive debulking, helps in definition of the
archnoidal plane. Operative microscope is mandatory to stay within
the archnoidal plane. The best way to free the adherent arteries
is to begin the dissection at uninvolved segments of the vessels.
Once
the tumor is excised, the involved dura and the bone are excised as
well and duraplasty with pericranium or temporalis fascia is carried
out. Unresectable dura should be aggressively cauterised.
Calvarial cranioplasty is better deferred as a later procedure to
accommodate post operative edema.
Considerations by tumor location:
Convexity meningiomas offer the
greatest potential for total tumor removal with a wide dural margin.
A circumferential dural incision around the tumor insertion allows
for early devascularization in the tumor. Central debulking helps in
accessing the arachnoidal plane.
In
Parasagittal and Falcine meningiomas, their proximity
to, and the extent of involvement to the sagittal sinus and the
draining cerebral veins must be considered. Tumor invasion anterior
to coronal suture may be managed with sinus ligation and excision.
Excision of patent sagittal sinus, posterior to coronal suture
carries significant risk of morbidity and mortality. Tumors attached
to the lateral wall, without significant infiltration into the sinus
lumen, can be managed by dissecting the tumor off the sinus and
achieving hemostasis by a combination of coagulation and pressure
over surgicel and gelfoam. If the tumor has infiltrated the
sinus lumen in the lateral aspect only, it may be excised and the
sinus progressively closed with a continuous running suture.
Excision of the sinus followed by repair with autogenous venous
grafts is being increasingly practiced. It is prudent to perform a
near total tumor resection, leaving the involved sinus
undisturbed. Utmost care is taken when dissecting at depth to
avoid injury to anterior cerebral arteries. Every effort should be
made to preserve large cortical veins. Extensive tumor debulking
avoids excessive brain retraction. Inferior sagital sinus is usually
involved in falcine meningiomas and may be excised.
The
anterior and middle skull base tumors may extend to several
intra and extracranial compartments. Orbital and/or zygomatic
osteotomies and other more
extensive skull base approaches may be needed to allow a more
basal approach to minimize brain retraction, and also help clear the
involved bone and the dura of the skull base. Continuous CSF drainage
though a lumbar catheter may obviate the need for brain
retraction.
Tuberculum
sella meningiomas displace the optic chiasm back and the optic
nerves laterally and superiorly; carotid artery may be found medial
to the displaced optic nerve. The pituitary stalk is posterior to the
tumor along the membrane of Lilliequist, which separates the tumor
from the neurovascular structures of the posterior fossa. Optic
deroofing may be required to remove tumor extension. Olfactory
Groove Meningiomas arise more anteriorly, and push the optic
chiasm and optic nerves dowm. Large tumors may require a midline a
bilateral bone flap. The anterior end of the sagittal sinus may
be ligated and the falx cerebri detached from its inferior attachment
when indicated. Any extension of the tumor into the air sinuses can
be removed by a frontobasal approach or a combined craniofacial
approach. Meticulous repair of the anterior cranial
fossa is necessary to prevent CSF rhinorrhoea. The approach may be
modified for other suprasellar meningiomas. They may derive
blood supply from the branches of the anterior cerebral artery and
anterior communicating complex. They must be traced to the tumor
prior to sacrifice.
Total
excision of medial sphenoid wing meningiomas, especially those
with significant involvement of the cavernous sinus, though not
impossible, is usually associated with significant morbidity.
Moreover, whether the patient really experiences long term benefits
from more extensive surgery and the increased risk of surgery, or,
whether partial removal of the tumor followed by radiotherapy is
better, is still debated. The ICA and its branches, as well as the
optic, oculomotor, and olfactory nerves are at risk. The
ophthalmic artery crosses the anterior corner of the opticocarotid
triangle, and its location must be anticipated. The anterior clinoid
meningiomas usually extend into cavernous sinus. Attempts at radical
excision of the tumor in the SOF usually results in
ophthalmoplegia. Hence, a more conservative alternative is excision
of the intracranial mass followed by radiosurgery or periodic
observation.
Pterional
Meningiomas are usually easily achieved with
careful microdissection of the branches of the MCA. The uncommon, meningioma
en plaque, is approached through a frontotemporal extradural
route. The hyperostotic posterolateral wall of the orbit needs to be
drilled out exposing the periorbita and frontotemporal dura.
Bone above and below the superior orbital fissure, over the optic
canal, anterior clinoid process, roof of the orbit and floor of the
middle cranial fossa may also need to be removed depending on the
extent of the lesion. Dural excision and intradural tumor
removal completes the surgical exercise. Careful reconstruction
of the dural and bone defect is essential. This extensive
surgery should be contemplated with utmost caution, as it is rarely
possible to completely eradicate the tumor and moreover, some
patients may develop visual deterioration following surgery.
The decision
regarding surgery in Cavernous Sinus Meningiomas depends on
the age and general condition of the patient and whether relief from
symptoms can be provided by operative treatment. Recent advances in
microsurgical skull base techniques have made total
excision of these tumors invading the cavernous sinus feasible with
reconstruction of the internal carotid artery by a bypass graft.
Medial
tentorial meningiomas can be approached by various routes
depending on their disposition in the longitudinal axis. An
anteriorly located tumor can be managed by either a frontotemporal
approach, extended anterior temporal approach with an anterior
temporal lobectomy or by a subtemporal approach. In large
tumours, it may be better to sacrifice a part of the inferior
temporal gyrus, to avoid excessive retraction and contusion of the
temporal lobe while employing the subtemporal approach. The vein of
Labbe should be protected at all costs to prevent temporal lobe
infarction. For more posterior medial tentorial tumors,
subtemporal approach is preferred.
Tentorial
apex
meningiomas are best approached by the occipital transtentorial
route. An alternative approach is the supracerebellar route
popularised. Torcular meningiomas are approached by either a
supra or infratentorial approach or a combined approach depending on
the extent of the tumor. Almost always a bilateral approach is
necessary. Unless the torcular Herophili is occluded completely
and adequate collaterals have developed, only subtotal excision is
advisable. Focal external cobalt beam irradiation of the
residual tumor is recommended in such an event.
The lateral
tentorial meningiomas are approached either by a subtemporal,
occipital, or temporoparietal route depending on the dominant
extension of the tumor. The main limiting factor for excision
of the posterolateral tumor is involvement of the transverse and
sigmoid sinuses. The main limiting factor for excision of the
posterolateral tumor is involvement of the transverse and sigmoid
sinuses. Total excision with ligation of the sinus is indicated
only in the presence of good torcular anastomosis and a patent
contralateral transverse sinus. In tumors with both supra and
infratentorial extensions either a subtemporal or a combined supra
and infratentorial approach is recommended. Though microsurgical
techniques have improved the results of surgery in these difficult
tumors, still there is significant morbidity associated with their
management, especially in medial tentorial tumors.
Cerebellar convexity in the
posterior fossa can be excised totally without significant problem,
except when the venous sinuses are involved. In the latter
instance, total excision of the tumor along with the involved sinus
can be achieved only if either the sinus is completely occluded or
the collaterals are well developed. Cerebellar convexity
meningiomas have a propensity to develop near the transverse-sigmoid
sinus junction and hence, sinus anatomy should be studied before
planning surgery.
CPAngle meningiomas are best excised
by the retrosigmoid approach in the lateral decubitus position. It is
beneficial to expose the presigmoid dura even during a retrosigmoid
approach so that the dura and the sigmoid sinus can be retracted
laterally, thus decreasing their obstruction of the surgeon’s view.
It is important to skeletonize the entire sinus from the tranverse
sinus junction to the jugular bulb in order to allow the full freedom
of movement of the sinus once the tentorium is sectioned. When the
tumor is huge with extensions into the tentorial hiatus, and the
parasellar region, or has a wide tentorial attachment, a combined
subtemporal and retromastoid approach or a petrosal approach may be
necessary.
Foramen magnum meningiomas,
especially, the ventral ones, pose a challenge to the surgeon, with a
high risk of morbidity. Various posterolateral approaches have been
recommended. Essentially it involves mobilizing the vertebral artery
medially and shaving off the outer third of the occipital condyle, so
that the surgeon will have unobstructed view. Good
microsurgical technique is mandatory, whichever approach is chosen.
Intratemporal meningiomas are better
approached by infratemporal approach of Fisch or one of
its many modifications. These tumors are inseparable from the
lower cranial nerves and hence it may be prudent to be satisfied with
subtotal excision rather than total excision with severe
postoperative morbidity. Some of these cases have en plaque
tumors over the petrous. In such cases, excision is hazardous
and unless the tumor is producing significant mass effect, it may be
periodically followed.
Intraventricular Meningiomas are
approached similar to that of other tumors in the same location. The
tumors in the trigone are best managed either by a parieto-occipital
approach or mid-temporal gyrus approach. Various other
approaches to the lateral ventricle have been described.
Post-operatively, as a rule,
basal meningiomas need much greater vigil than convexity meningiomas.
Postoperative
course is usually uneventful unless major veins have been
sacrificed. Patients with tumors located over the central
sulcus, even with no apparent venous disturbance at surgery, frequently
have transient post-operative focal deficit on the appropriate side
of the body. Post-operative epilepsy is frequent. Careful attention
is warranted to facilitate cotical venous drainage; mannitol is
avoided unless it becomes life saving and hypervolemic treatment
helps.
Patients
with a meningioma in the skull base may have a stormy post-operative
course, in spite of microsurgical techniques. The commonest
problem is that of a CSF leak with its associated
complications. A few days of prophylactic ventricular or lumbar
drainage may be useful. Cranial nerve paresis is not uncommon
and when the lower cranial nerves are involved, adequate care of the
airway with ventilatory support may be essential. A short
period of nasogastric tube feeding may be necessary.
Recurrent meningiomas:
Among
extra-axial brain tumors, meningiomas represent the largest group
capable of recurrence. These tumors may recur, either because
of incomplete removal or a true recurrence. The overall
recurrence rates range from 13-40 per cent.
The most
important factor in the recurrence of meningiomas was the extent of
removal.
|
Simpson classified the types of excision into five
grades and found that while the recurrence rate was nine per cent
in grade I excision (complete macroscopic tumor removal with
excision of involved dura and bone), it was 44 per cent in grade IV
excision (intracranial tumor was left in situ). This
relationship has been confirmed by other authors. As a meningioma
is a slow growing tumor, the risk is also directly related to the
length of follow up. Complete removal of a meningioma is not always
feasible and remnants of the tumor may be left behind in the dura,
involved bone, venous sinus wall or parts of the tumor adherent to
vital structures thus leading to recurrence.
|
|
|
Simpson’s Grading
|
|
|
|
GRADES
|
FEATURES
|
|
I
|
Complete
removal, including resection of dura and bone
|
|
II
|
Complete
tumor removal with coagulation of dural attachment.
|
|
III
|
Complete
tumor removal without resection or coagulation of dural
attachment.
|
|
IV
|
Subtotal
removal
|
|
V
|
Decompression
|
|
|
Gadolinium
MR can help to identify these dural extensions preoperatively to help
excision. These dural extensions could explain recurrence after
apparent complete tumor removal in a surface meningioma. It is
recommended that by removing an additional margin of two cm of dura
around the tumors and enbloc resection of hyperostotic bone with
ahealthy margin and the pericranium to prevent redrowth in convexity
meningiomas.
Studies
evaluating proliferative activity and tumor kinetics by the
argyrophilic method for the demonstration of nucleolar organizer
regions (Ag-NOR), flowcytometry, and bromodeoxyuridine labelling
index (BUdRLI) allow the detection of aggressive behavior in
meningiomas indicating regrowth potential. The recurrence rate is
significantly higher in atypical meningiomas than in other
histopathological types and it has been suggested that a higher
Ag-NOR count is suggestive of aggressive behaviour in meningiomas and
is associated with an increased risk of recurrence.
Other
factors like bone invasion and brain infiltration have been
considered important for the higher incidence of recurrence by some,
but not by others. It has been suggested that the recurrence is
more frequent in the younger age group; Some surgeons feel the age is
no predictor of recurrence.
Hence,
the decision for further therapy needs careful judgment. It is
not difficult to decide on reoperation in a patient with recurrence
in a resectable location and progressive symptomatology.
However, a small recurrence, especially in a difficult location like
the skull base or posterior half of the parasagittal region, may be
better followed periodically or, radiosurgery may be
considered. In case the patient becomes symptomatic with an
increase in the size of the tumor, a second operation is
justified. In cases following reoperation, those with an en
plaque tumor, and patients with anticipated problems at excision,
adjuvant radiotherapy may be considered. Hormone therapy with
anti-progesterone drugs may play a role in the future.
Radiation Therapy:
The
role of radiotherapy in meningiomas is controversial. Wara et
al, reported that after incomplete tumor removal the incidence of
recurrence at five years was 29 per cent in an irradiated group as
opposed to 74 per cent in non-irradiated patients. Radiation
has also been shown to improve survival in malignant meningiomas and
in incompletely resected and inoperable meningiomas of all three
histological types (benign, ‘aggressive benign’, malignant).
Stereotactic radiosurgery also may
have a role in patients with residual/recurrent tumors, in tumors in
high risk locations, and in patients who are unfit for surgery
because of age or an associated medical condition. Kondziolka
et al, in a recent review of the results of radiosurgery in
meningiomas, found that among 24 patients with 12-36 months follow
up, 54 per cent had a reduction in tumor volume and 38 per cent
showed no change. The actuarial two year tumor growth control
was 96 per cent. Between 3-12 months, three patients developed
neurological deficits because of delayed radiation injury.
However, an extended follow up is warranted, before any definite
conclusion can be drawn as to the effectiveness of radiosurgery.
In
the treatment of skull base meningiomas which have been incompletely
resected or have recurred, interstitial irradiation with I 125 seeds
has been found to be safe and effective. Preoperative radiotherapy in
vascular meningiomas administering 30 Gy or radiation makes surgical
excision feasible six weeks after radiation.
Chemotherapy:
Antagonism
of possible mitogenic hormones (estrogen and progesterone) has been
the main focus of chemotherapy of meningioma. There is little benefit
with Tamoxifen (40mg/m2 twice daily for 4 days and 10mg twice daily
thereafter) in unresectable or refractory meningiomas. Some benefit
with 200mg daily of Mifepristone (RU-486) has been reported. Post
operative radiotherapy and 3-6 weeks of chemotherapy with
cyclophosphamide, adriamycin and vincristine, reportedly, improves
survival. A high affinity dopamine D1 binding sites in meningioma
tissue using kinetic studies have been reported. Bromocriptine is
found to have some inhibitory effect on meningiomas. dFurther studies are
required.
The future:
It
is well established that the patient with a meningioma has a high
frequency of chromosome 22 monosomy (72%) and frequently has deletion
of the long arm of chromosome 22. Recent studies have
demonstrated specific loss of chromosome 22 markers. It is therefore
believed that meningioma growth is due to loss of the tumor
suppressor gene or the anti-oncogene at the LIF locus. The
exact gene has still to be identified, clones and the apparatus that
controls the gene deciphered, which would then lead to another major
insight into the etiology of meningiomas and it would be possible to
develop diagnostic and therapeutic strategies likely to revolutionize
the management of these neoplasms.
Oncogenes
have also been found in meningiomas and DNA coding for both EGF
receptors and PGF have been found. The DNA studies are developing
very fast and application of these techniques in molecular meningioma
studies increases the insight in meningioma pathogenesis.
The relationship of sex hormones and meningioma
has been known since Cushing, who noted that meningiomas had
increased
growth during pregnancy, and the relationship
between the breast carcinoma and meningioma is also well-known.
Recent studies
have demonstrated progesterone receptors and
oestrogen receptors. Receptor activity for progesterone has
been demonstrated,
but receptor activity for oestrogen has not been
demonstrated. In studies of growth characteristics of meningiomas
bromodeoxyuridase have been used and shown to be
more sensitive than mitotic index in distinguishing a group of
histologically
benign tumors form malignant tumors and thereby
are a better guide for the best treatment and follow-up of a
meningioma patient.
|